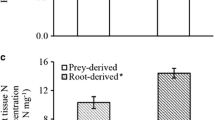
Nutritional factors are among the most important influences on primate food choice. We examined the influence of macronutrients, minerals, and secondary compounds on leaf choices by members of a foli-frugivorous population of eastern black-and-white colobus—or guerezas (Colobus guereza)—inhabiting the Kakamega Forest, Kenya. Macronutrients exerted a complex influence on guereza leaf choice at Kakamega. At a broad level, protein content was the primary factor determining whether or not guerezas consumed specific leaf items, with eaten leaves at or above a protein threshold of ca. 14% dry matter. However, a finer grade analysis considering the selection ratios of only items eaten revealed that fiber played a much greater role than protein in influencing the rates at which different items were eaten relative to their abundance in the forest. Most minerals did not appear to influence leaf choice, though guerezas did exhibit strong selectivity for leaves rich in zinc. Guerezas avoided most leaves high in secondary compounds, though their top food item (Prunus africana mature leaves) contained some of the highest condensed tannin concentrations of any leaves in their diet. Kakamega guerezas periodically traveled great distances to exploit rare foods (bark from exotic Myrtaceae trees and soil) outside their normal home ranges. Our results suggest that these journeys were driven by the fact that these rare foods contained exceptionally high sodium concentrations, a mineral believed to be deficient in the guereza's usual diet. Lastly, our results are consistent with the pattern established across other Paleotropical rain forests in which colobine biomass can be predicted by the protein-to-fiber ratio in mature leaves. Of the 8 rain forests for which the relevant data are available, Kakamega features the second highest mature leaf protein-to-fiber ratio as well as the second highest colobine biomass.



Similar content being viewed by others
REFERENCES
Altmann, S. A. (1991). Diets of yearling female primates (Papio cynocephalus) predict lifetime fitness. Proc. Natl. Acad. Sci. USA 88: 420–423.
Altmann, S. A. (1998). Foraging for Survival: Yearling Baboons in Africa. University of Chicago Press, Chicago.
Baranga, D. (1982). Nutrient composition and food preferences of colobus monkeys in Kibale Forest, Uganda. Afr. J. Ecol. 20: 113–121.
Baranga, D. (1983). Changes in chemical composition of food parts in the diet of colobus monkeys. Ecology 64: 668–673.
Bate-Smith, E. C., and Metacalfe, C. R. (1957). Leuco-anthocyanins 3. The nature and systematic distribution of tannins in dicotyledonous plants. J. Linn. Soc. (Bot.) 55: 669–705.
Bauchop, T. (1978). Digestion of leaves in vertebrate arboreal folivores. In Montgomery, G. G. (ed.), The Ecology of Arboreal Folivores. Smithsonian Institution Press, Washington, D.C., pp. 193–204.
Bauchop, T., and Martucci, R. W. (1968). Ruminant-like digestion of the langur monkey. Science 161: 698–700.
BIOTA. (2004). Biodiversity in Conservation: Influence of Fragmentation and Disturbance on the Biodiversity of East African Highland Forests. Final Report Phase I (2001–2004), BIOTA East Africa.
Bocian, C. M. (1997). Niche Separation of Black-and-White Colobus Monkeys (Colobus angolensis and C. guereza) in the Ituri Forest. Ph.D. thesis, City University of New York, New York.
Botkin, D. B., Jordan, P. A., Dominski, A. S., Lowendor, H. S., and Hutchins, G. E. (1973). Sodium dynamics in a northern ecosystem. Proc. Natl. Acad. Sci. USA 70: 2745–2748.
Bryant, J. P., Reichardt, P. B., Clausen, T. P., Provenza, F. D., and Kuropata, P. J. (1992). Woody plant-mammal interactions. In Rosental, G. A., and Berenbaum, M. R. (eds.), Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press, New York.
Burgess, M. A., and Chapman, C. A. (2005). Tree leaf chemical characters: Selective pressures by folivorous primates and invertebrates. Afr. J. Ecol. 43: 242–250.
Chapman, C. A., and Balcomb, S. R. (1998). Population characteristics of howlers: Ecological conditions or group history. Int. J. Primatol. 19: 385–403.
Chapman, C. A., and Chapman, L. J. (2002). Foraging challenges of red colobus monkeys: Influence of nutrients and secondary compounds. Comp. Biochem. Physiol. A: 133: 861– 875.
Chapman, C. A., Chapman, L. J., Bjorndal, K. A., and Onderdonk, D. A. (2002). Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. Int. J. Primatol. 23: 283–310.
Chapman, C. A., Chapman, L. J., Naughton-Treves, L., Lawes, M. J., and McDowell, L. R. (2004). Predicting folivorous primate abundance: Validation of a nutritional model. Am. J. Primatol. 62: 55–69.
Chapman, C. A., Chapman, L. J., Rode, K. D., Hauck, E. M., and McDowell, L. R. (2003). Variation in the nutritional value of primate foods: Among trees, time periods, and areas. Int. J. Primatol. 24: 317–333.
Chivers, D. J. (1994). Functional morphology of the gastrointestinal tract. In Davies, A. G., and Oates, J. F. (eds.), Colobine Monkeys: Their Ecology, Behaviour and Evolution, Cambridge University Press, Cambridge, UK, pp. 205–228.
Coley, P. D. (1983). Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol. Monogr. 53: 209–233.
Cords, M. (1987). Mixed-Species Association of Cercopithecus Monkeys in the Kakamega Forest, Kenya. University of California Publications in Zoology, Vol. 17. University of California Press, Berkeley.
Crissey, S. D., and Pribyl, L. S. (1997). Utilizing wild foraging ecology information to provide captive primates with an appropriate diet. Proc. Nutr. Soc. 56: 1083–1094.
Cunniff, P. A. (ed.). (1996). Official Methods of Analysis of AOAC International, 16th ed. AOAC International, Gaithersberg, MD.
Dasilva, G. L. (1992). The western black-and-white colobus as a low-energy strategist: Activity budgets, energy-expenditure and energy-intake. J. Anim. Ecol. 61: 79–91.
Dasilva, G. L. (1994). Diet of Colobus polykomos on Tiwai Island: Selection of food in relation to its seasonal abundance and nutritional quality. Int. J. Primatol. 15: 655–680.
Davies, A. G. (1994). Colobine populations. In Davies, A. G., and Oates, J. F. (eds.), Colobine Monkeys: Their Ecology, Behaviour and Evolution, Cambridge University Press, Cambridge, UK, pp. 285–310.
Davies, A. G., and Baillie, I. C. (1988). Soil-eating by red leaf monkeys (Presbytis rubicunda) in Sabah, Northern Borneo. Biotropica 20: 252–258.
Davies, A. G., Bennett, E. L., and Waterman, P. G. (1988). Food selection by two South-East Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biol. J. Linn. Soc. 34: 33–56.
Dierenfeld, E. S., and McCann, C. M. (1999). Nutrient composition of selected plant species consumed by semi free-ranging lion-tailed macaques (Macaca silenus) and ring-tailed lemurs (Lemur catta) on St. Catherines Island, Georgia, USA. Zoo Biol. 18: 481–494.
Fashing, P. J. (1999). The Behavioral Ecology of an African Colobine Monkey: Diet, Range Use, and Patterns of Intergroup Aggression in Eastern Black and White Colobus Monkeys (Colobus guereza). Ph.D. thesis, Columbia University, New York.
Fashing, P. J. (2001a). Activity and ranging patterns of guerezas in the Kakamega Forest: Intergroup variation and implications for intragroup feeding competition. Int. J. Primatol. 22: 549–577.
Fashing, P. J. (2001b). Feeding ecology of guerezas in the Kakamega Forest, Kenya: The importance of Moraceae fruit in their diet. Int. J. Primatol. 22: 579–609.
Fashing, P. J. (2004). Mortality trends in the African cherry (Prunus africana) and the implications for colobus monkeys (Colobus guereza) in Kakamega Forest, Kenya. Biol. Conserv. 120: 449–459.
Fashing, P. J. (2006). African colobine monkeys: Patterns of between-group interaction. In Campbell, C. J., Fuentes, A., MacKinnon, K., Panger, M., and Bearder, S. K. (eds.), Primates in Perspective. Oxford University Press, Oxford, pp. 201–224.
Fashing, P. J., and Cords, M. (2000). Diurnal primate densities and biomass in the Kakamega Forest: An evaluation of census methods and a comparison with other forests. Am. J. Primatol. 50: 139–152.
Fashing, P. J., Forrestel, A., Scully, C., and Cords, M. (2004). Long-term tree population dynamics and their implications for the conservation of the Kakamega Forest, Kenya. Biodivers. Conserv. 13: 753–771.
Freeland, W. J., Calcott, P. H., and Anderson, L. R. (1985). Tannins and saponin: Interaction in herbivore diets. Biochem. Syst. Ecol. 13: 189–193.
Freeland, W. J., and Janzen, D. H. (1974). Strategies in herbivory by mammals: Role of plant secondary compounds. Am. Nat. 108: 269–289.
Ganzhorn, J. U. (1992). Leaf chemistry and the biomass of folivorous primates in tropical forests: Test of a hypothesis. Oecologia 91: 540–547.
Gartlan, J. S., McKey, D. B., Waterman, P. G., Mbi, C. N., and Struhsaker, T. T. (1980). A comparative study of the phytochemistry of two African rain forests. Biochem. Syst. Ecol. 8: 401–422.
Gaulin, S. J. C. (1979). A Jarman/Bell model for primate feeding. Hum. Ecol. 7: 1–20.
Glander, K. E. (1982). The impact of plant secondary compounds on primate feeding behavior. Yearb. Phys. Anthropol. 25: 1–18.
Harding, R. S. O. (1981). An order of omnivores: Nonhuman primate diets in the wild. In Harding, R. S. O., and Teleki, G. (eds.), Omnivorous Primates. Columbia University Press, New York, pp. 191–214.
Harris, T. R. (2005). Roaring, Intergroup Aggression, and Feeding Competition in Black and White Colobus Monkeys (Colobus guereza) at Kanyawara, Kibale National Park, Uganda. Ph.D. thesis, Yale University, New Haven.
Hladik, C. M. (1978). Adaptive strategies of primates in relation to leaf-eating. In Montgomery, G. G. (ed.), The Ecology of Arboreal Folivores. Smithsonian Institution Press, Washington, D.C., pp. 373–395.
Janson, C. H. (1988). Intraspecific food competition and primate social structure: A synthesis. Behaviour 105: 1–17.
Kay, R. N. B., and Davies, A. G. (1994). Digestive physiology. In Davies, A. G., and Oates, J. F. (eds.), Colobine Monkeys: Their Ecology, Behaviour, and Evolution, Cambridge University Press, Cambridge, UK, pp. 229–249.
King, J. C., and Keen, C. L. (1999). Zinc. In Shils, M. E., Olson, J. A., Shike, M., and Ross, A. C. (eds.), Modern Nutrition in Health and Disease. Lippincott, Williams & Wilkins, Philadelphia, pp. 223–239.
Kirkpatrick, R. C. (1998). Ecology and behavior of snub-nosed and douc langurs. In Jablonski, N. G. (ed.), The Natural History of the Doucs and Snub-Nosed Monkeys. World Scientific Press, Singapore, pp. 155–190.
Kirkpatrick, R. C. (2006). The Asian colobines: Diversity among leaf-eating monkeys. In Campbell, C. J., Fuentes, A., MacKinnon, K., Panger, M., and Bearder, S. K. (eds.), Primates in Perspective. Oxford University Press, Oxford, pp. 186–200.
Klaus, G., and Schmid, B. (1998). Geophagy at natural licks and mammal ecology: A review. Mammalia 62: 481–497.
Kool, K. M. (1989). Behavioural Ecology of the Silver Leaf Monkey, Trachypithecus auratus sondaicus, in the Pangandaran Nature Reserve, West Java, Indonesia. Ph.D. thesis, University of New South Wales, Sydney.
Kool, K. M. (1992). Food selection by the silver leaf monkey, Trachypithecus auratus sondaicus, in relation to plant chemistry. Oecologia 90: 527–533.
Kool, K. M. (1993). The diet and feeding behavior of the silver leaf monkey (Trachypithecus auratus sondaicus) in Indonesia. Int. J. Primatol. 14: 667–700.
Lambert, J. E. (1998). Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evol. Anthropol. 7: 8–20.
Levin, D. A. (1976). Chemical defenses of plants to pathogens and herbivores. Annu. Rev. Ecol. Syst. 7: 121–159.
Maisels, F., Gauthierhion, A., and Gautier, J. P. (1994). Diets of two sympatric colobines in Zaire: More evidence on seed-eating in forests on poor soils. Int. J. Primatol. 15: 681–701.
Marsh, C. W. (1981). Diet choice among red colobus (Colobus badius rufomitratus) on the Tana River, Kenya. Folia Primatol. 35: 147–178.
McKey, D. (1978). Soils, vegetation and seed-eating by black colobus monkeys. In Montgomery, G. G. (ed.), The Ecology of Arboreal Folivores. Smithsonian Institution Press, Washington, D.C., pp. 423–437.
McKey, D. B., Gartlan, J. S., Waterman, P. G., and Choo, G. M. (1981). Food selection by black colobus monkeys (Colobus satanos) in relation to food chemistry. Biol. Linnean Soc. 16: 115–146.
Miller, L. E. (2002). Eat or Be Eaten: Predator Sensitive Foraging in Primates. Cambridge University Press, Cambridge, UK.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: Test of some hypotheses of food selection by generalist herbivores. Am. Nat. 114: 362–378.
Milton, K. (1993). Diet and primate evolution. Sci. Am. 269: 86–93.
Milton, K. (1998). Physiological ecology of howlers (Alouatta): Energetic and digestive considerations and comparison with the Colobinae. Int. J. Primatol. 19: 513–548.
Milton, K. (2003). Micronutrient intakes of wild primates: Are humans different? Comp. Biochem. Physiol. A 136: 47–59.
Mowry, C. B., Decker, B. S., and Shure, D. J. (1996). The role of phytochemistry in dietary choices of Tana River red colobus monkeys (Procolobus badius rufomitratus). Int. J. Primatol. 17: 63–84.
National Research Council. (2003). Nutrient Requirements of Nonhuman Primates, 2nd revised edition. National Academy of Sciences Press, Washington, D.C.
Oates, J. F. (1977a). The guereza and its food. In Clutton-Brock, T. H. (ed.), Primate Ecology. Academic Press, London, pp. 276–321.
Oates, J. F. (1977b). Social life of a black-and-white colobus monkey, Colobus guereza. Z. Tierpsychol. 45: 1–60.
Oates, J. F. (1978). Water plant and soil consumption by guereza monkeys (Colobus guereza): Relationship with minerals and toxins in the diet. Biotropica 10: 241–253.
Oates, J. F. (1987). Food distribution and foraging behavior. In Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies. University of Chicago Press, Chicago, pp. 197–209.
Oates, J. F. (1988). The diet of the olive colobus monkey, Procolobus verus, in Sierra Leone. Int. J. Primatol. 9: 457–478.
Oates, J. F., and Davies, A. G. (1994). What are the colobines? In Davies, A. G., and Oates, J. F. (eds.), Colobine Monkeys: Their Ecology, Behaviour, and Evolution. Cambridge University Press, Cambridge, UK, pp. 1–10.
Oates, J. F., Swain, T., and Zantovska, J. (1977). Secondary compounds and food selection by colobus monkeys. Biochem. Syst. Ecol. 5: 317–321.
Oates, J. F., Waterman, P. G., and Choo, G. M. (1980). Food selection by the South Indian leaf-monkey, Presbytis johnii, in relation to leaf chemistry. Oecologia 45: 45–56.
Oates, J. F., Whitesides, G. H., Davies, A. G., Waterman, P. G., Green, S. M., Dasilva, G. L., and Mole, S. (1990). Determinants of variation in tropical forest primate biomass: New evidence from West Africa. Ecology 71: 328–343.
Pages, G., Lloyd, E., and Suarez, S. A. (2005). The impact of geophagy on ranging behaviour in Phayre's leaf monkeys (Trachypithecus phayrei). Folia Primatol. 76: 342–346.
Poulsen, J. R., Clark, C. J., Connor, E. F., and Smith, T. B. (2002). Differential resource use by primates and hornbills: Implications for seed dispersal. Ecology 83: 228–240.
Powzyk, J. A., and Mowry, C. B. (2003). Dietary and feeding differences between sympatric Propithecus diadema diadema and Indri indri. Int. J. Primatol. 24: 1143–1162.
Pyke, G. H., Pulliam, H. R., and Charnov, E. L. (1977). Optimal foraging: Selective review of theory and tests. Q. Rev. Biol. 52: 137–154.
Remis, M. J., Dierenfeld, E. S., Mowry, C. B., and Carroll, R. W. (2001). Nutritional aspects of western lowland gorilla (Gorilla gorilla gorilla) diet during seasons of fruit scarcity at Bai Hokou, Central African Republic. Int. J. Primatol. 22: 807–836.
Richard, A. F. (1985). Primates in Nature. W.H. Freeman, New York.
Rode, K. D., Chapman, C. A., Chapman, L. J., and McDowell, L. R. (2003). Mineral resource availability and consumption by colobus in Kibale National Park, Uganda. Int. J. Primatol. 24: 541–573.
Rogers, M. E., Maisels, F., Williamson, E. A., Fernandez, M., and Tutin, C. E. G. (1990). Gorilla diet in the Lope Reserve, Gabon: A nutritional analysis. Oecologia 84: 326–339.
Silver, S. C., Ostro, L. E. T., Yeager, C. P., and Dierenfeld, E. S. (2000). Phytochemical and mineral components of foods consumed by black howler monkeys (Alouatta pigra) at two sites in Belize. Zoo Biol. 19: 95–109.
Stanford, C. B. (1991). The diet of the capped langur (Presbytis pileata) in a moist deciduous forest in Bangladesh. Int. J. Primatol. 12: 199–216.
Starin, E. D. (1991). Socioecology of the Red Colobus Monkey in the Gambia with Particular Reference to Female-Male Differences and Transfer Patterns. Ph.D. thesis, City University of New York, New York.
Stephens, D. W., and Krebs, J. R. (1986). Foraging Theory. Princeton University Press, Princeton, NJ.
Struhsaker, T. T. (1975). The Red Colobus Monkey. University of Chicago Press, Chicago.
Struhsaker, T. T., Cooney, D. O., and Siex, K. S. (1997). Charcoal consumption by Zanzibar red colobus monkeys: Its function and its ecological and demographic consequences. Int. J. Primatol. 18: 61–72.
van Noordwijk, M. A., and van Schaik, C. P. (1987). Competition among female long-tailed macaques, Macaca fascicularis. Anim. Behav. 35: 577589.
van Noordwijk, M. A., and van Schaik, C. P. (1999). The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates 40: 105–130.
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74: 3583–3597.
Waser, P. M. (1987). Interactions among primate species. In Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies. University of Chicago Press, Chicago, pp. 210–226.
Wasserman, M. D., and Chapman, C. A. (2003). Determinants of colobine monkey abundance: The importance of food energy, protein and fibre content. J. Anim. Ecol. 72: 650–659.
Waterman, P. G., and Kool, K. M. (1994). Colobine food selection and plant chemistry. In Davies, A. G., and Oates, J. F. (eds.), Colobine Monkeys: Their Ecology, Behaviour, and Evolution. Cambridge University Press, Cambridge, UK, pp. 251–284.
Waterman, P. G., Ross, J. A. M., Bennett, E. L., and Davies, A. G. (1988). A comparison of the floristics and leaf chemistry of the tree flora in two Malaysian rain forests and the influence of leaf chemistry on populations of colobine monkeys in the Old World. Biol. J. Linn. Soc. 34: 1–32.
Watkins, B. E., Ullrey, D. E., and Whetter, P. A. (1985). Digestibility of a high-fiber biscuit-based diet by black and white colobus (Colobus guereza). Am. J. Primatol. 9: 137–144.
Westoby, M. (1974). Analysis of diet selection by large generalist herbivores. Am. Nat. 108: 290–304.
Whitten, P. L. (1983). Diet and dominance among female vervet monkeys (Cercopithecus aethiops). Am. J. Primatol. 5: 139–159.
Williams-Guillen, K. (2003). Behavioral Ecology of Mantled Howling Monkeys (Alouatta palliata) in a Coffee Plantation in Nicaragua. Ph.D. thesis, New York University, New York.
Worman, C. O., and Chapman, C. A. (2005). Seasonal variation in the quality of a tropical ripe fruit and the response of three frugivores. J. Trop. Ecol. 21: 689–697.
Wrangham, R. W., and Waterman, P. G. (1983). Condensed tannins in fruits eaten by chimpanzees. Biotropica 15: 217–222.
Yeager, C. P., Silver, S. C., and Dierenfeld, E. S. (1997). Mineral and phytochemical influences on foliage selection by the proboscis monkey (Nasalis larvatus). Am. J. Primatol. 41: 117–128.
ACKNOWLEDGMENTS
We thank Berry College, Columbia University, Gisela and Norman Fashing, Leakey Foundation, Pittsburgh Zoo, Wenner-Gren Foundation, and Wildlife Conservation Society for financial support of this research. We thank the Kenyan government for research permission and the Institute of Primate Research for local sponsorship. We thank Saphalinas Imboma, Emily Mujinji, Benjamin Okalo, Wilberforce Okeka, Radhika Shah, and tree climbers from KEFRI for their assistance in the field. We thank Irine Rudik and colleagues in the Animal Toxicology Lab at the University of Pennsylvania for conducting the mineral analyses and Jasmine Thomas for assisting with the macronutrient analyses. Colin Chapman, Nga Nguyen, and an anonymous reviewer kindly read and commented on an earlier draft of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fashing, P.J., Dierenfeld, E.S. & Mowry, C.B. Influence of Plant and Soil Chemistry on Food Selection, Ranging Patterns, and Biomass of Colobus guereza in Kakamega Forest, Kenya. Int J Primatol 28, 673–703 (2007). https://doi.org/10.1007/s10764-006-9096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-006-9096-2