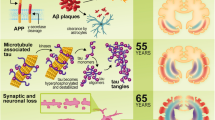
Abstract
Neurodegenerative diseases such as Alzheimer, Parkinson, amyotrophic lateral sclerosis, and Huntington are incurable and debilitating conditions that result in progressive death of the neurons. The definite diagnosis of a neurodegenerative disorder is disadvantaged by the difficulty in obtaining biopsies and thereby to validate the clinical diagnosis with pathological results. Biomarkers are valuable indicators for detecting different phases of a disease such as prevention, early onset, treatment, progression, and monitoring the effect of pharmacological responses to a therapeutic intervention. Inflammation occurs in neurodegenerative diseases, and identification and validation of molecules involved in this process could be a strategy for finding new biomarkers. The ideal inflammatory biomarker needs to be easily measurable, must be reproducible, not subject to wide variation in the population, and unaffected by external factors. Our review summarizes the most important inflammation biomarkers currently available, whose specificity could be utilized for identifying and monitoring distinctive phases of different neurodegenerative diseases.

Similar content being viewed by others
References
Glass, C.K., K. Saijo, B. Winner, M.C. Marchetto, and F.H. Gage. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140(6): 918–934.
Amor, S., F. Puentes, D. Baker, and P. van der Valk. 2010. Inflammation in neurodegenerative disease. Immunology 129: 154–169.
Zhang, Y.Y., Y.C. Fan, M. Wang, D. Wang, and X.H. Li. 2013. Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-α in the hippocampus of an amyloid β1-42-induced rat model of Alzheimer’s disease. Clinical Interventions in Aging 8: 103–110.
Nolan, Y.M., Sullivan, A.M., Toulouse, A. (2013) Parkinson’s disease in the nuclear age of neuroinflammation. Trends in Molecular Medicine, 19, 187–196.
Hsiao, H.Y., Chen, Y.C., Chen, H.M., Tu, P.H., Chern, Y. (2013) A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in Huntington’s disease. Human Molecular Genetics, 22, 1826–1842.
Wyss-Coray, T. 2006. Inflammation in Alzheimer disease: driving force bystander or beneficial response? Nature Medicine 12: 1005–1015.
Singh, S., A.S. Kushwah, R. Singh, M. Farswan, and R. Kaur. 2012. Current therapeutic strategy in Alzheimer’s disease. European Review for Medical and Pharmacological Sciences 16(12): 1651–1664.
Aluise, C.D., R.A. Sowell, and D.A. Butterfield. 2008. Peptides and proteins in plasma and cerebro spinal fluid as biomarkers for the prediction, diagnosis, and monitoring of therapeutic efficacy of Alzheimer’s disease. Biochimica et Biophysica Acta 1782(10): 549–558.
Reitz, C., C. Brayne, and R. Mayeux. 2011. Epidemiology of Alzheimer disease. Nature Reviews Neurology 7(3): 137–152.
Hebert, L.E., Weuve, J., Scherr, P.A., Evans, D.A. (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80, 1778–1783.
Guerreiro, R.J., D.R. Gustafson, and J. Hardy. 2012. The genetic architecture of Alzheimer’s disease: beyond APP, PSENs and APOE. Neurobiology of Aging 33(3): 437–456.
McKhann, G.M., D.S. Knopman, H. Chertkow, B.T. Hyman, C.R. Jack Jr., C.H. Kawas, W.E. Klunk, W.J. Koroshetz, J.J. Manly, R. Mayeux, R.C. Mohs, J.C. Morris, M.N. Rossor, P. Scheltens, M.C. Carrillo, B. Thies, S. Weintraub, and C.H. Phelps. 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers & Dementia 7(3): 263–269.
Wyss-Coray, T., and J. Rogers. 2012. Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine 2(1): a006346.
Balistreri, C.R., G. Colonna-Romano, D. Lio, G. Candore, and C. Caruso. 2009. TLR4 polymorphisms and ageing: implications for the pathophysiology of age-related diseases. Journal of Clinical Immunology 29(4): 406–415.
Mrak, R.E., and W.S.T. Griffin. 2005. Potential inflammatory biomarkers in Alzheimer’s disease. Journal of Alzheimer’s Disease 8(4): 369–375.
Harigaya, Y., M. Shoji, T. Nakamura, E. Matsubara, K. Hosoda, and S. Hirai. 1995. Alpha 1-antichymotrypsin level in cerebrospinal fluid is closely associated with late onset Alzheimer’s disease. Internal Medicine 34(6): 481–484.
Pirttila, T., P.D. Mehta, H. Frey, and H.M. Wisniewski. 1994. α1Antichymotrypsin and IL-1β are not increased in CSF or serum in Alzheimer’s disease. Neurobiology of Aging 15(3): 313–317.
Peskind, E.R., W.S. Griffin, K.T. Akama, M.A. Raskind, and L.J. Van Eldik. 2001. Cerebrospinal fluid s100B is elevated in the earlier stages of Alzheimer’s disease. Neurochemistry International 39: 409–413.
Petzold, A., R. Jenkins, H.C. Watt, A.J. Green, E.J. Thompson, G. Keir, N.C. Fox, and M.N. Rossor. 2003. Cerebrospinal fluid s100B correlates with brain atrophy in Alzheimer’s disease. Neuroscience Letters 336: 167–170.
Xia, M., S. Qin, M. McNamara, C. Mackay, and B.T. Hyman. 1997. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. American Journal of Pathology 150(4): 1267–1274.
Zhang, J., I. Sokal, E.R. Peskind, J.F. Quinn, J. Jankovic, C. Kenney, K.A. Chung, S.P. Millard, J.G. Nutt, and T.J. Montine. 2008. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. American Journal of Clinical Pathology 129(4): 526–529.
Zuliani, G., G. Guerra, M. Ranzini, L. Rossi, M.R. Munari, A. Zurlo, A. Blè, S. Volpato, A.R. Atti, and R. Fellin. 2007. High interleukin-6 plasma levels are associated with functional impairment in older patients with vascular dementia. International Journal of Geriatric Psychiatry 22(4): 305–331.
Luterman, J.D., V. Haroutunian, S. Yemul, et al. 2000. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Archives of Neurology 57(8): 1153–1160.
Zhang, J., D. Goodlett, J. Quinn, et al. 2005. Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer’s disease. Journal of Alzheimers Disease 7: 125–133.
Hu, Y., A. Hosseini, J. Kauwe, et al. 2007. Identification and validation of novel CSF biomarkers for early stages of Alzheimer’s disease. Proteomics Clinical Applications 1: 1373–1384.
Castano, E., A. Roher, C. Esh, T. Kokjohn, and T. Beach. 2006. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurological Research 28: 155–163.
Lewczuk, P., and J. Wiltfang. 2008. Neurochemical dementia diagnostics: state of the art and research perspectives. Proteomics 8: 1292–1301.
Ray, S., M. Britschgi, C. Herbert, Y. Takeda-Uchimura, A. Boxer, K. Blennow, L.F. Friedman, D.R. Galasko, M. Jutel, A. Karydas, et al. 2007. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nature Medicine 13: 1359–1362.
Doecke, J.D., S.M. Laws, N.G. Faux, W. Wilson, S.C. Burnham, C.P. Lam, A. Mondal, J. Bedo, A.I. Bush, B. Brown, K. De Ruyck, K.A. Ellis, C. Fowler, V.B. Gupta, R. Head, S.L. Macaulay, K. Pertile, C.C. Rowe, A. Rembach, M. Rodrigues, R. Rumble, C. Szoeke, K. Taddei, T. Taddei, B. Trounson, D. Ames, C.L. Masters, and R.N. Martins. 2012. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Archives of Neurology 69(10): 1318–1325.
Soares, D.H., et al. 2012. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Archives of Neurology 69: 1310–1317.
Henkel, A.W., K. Muller, P. Lewczuk, T. Muller, K. Marcus, J. Kornhuber, and J. Wiltfang. 2012. Multidimensional plasma protein separation technique for identification of potential Alzheimer’s disease plasma biomarkers: a pilot study. Journal of Neural Transmission 119: 779–788.
Crosiers, D., J. Theuns, P. Cras, and C. Van Broeckhoven. 2011. Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. Journal of Chemical Neuroanatomy 42(2): 131–141.
Reichmann, H. 2011. View point: etiology in Parkinson’s disease. Dual hit or spreading intoxication. Journal of the Neurological Sciences 310(1–2): 9–11.
Lesage, S., and A. Brice. 2012. Role of Mendelian genes in “sporadic” Parkinson’s disease. Parkinsonism & Related Disorders 18(supplement 1): S66–S70.
Taccioli, C., Maselli, V., Tegn’er, J., et al. (2011) ParkDB: a Parkinson’s disease gene expression database. Database, vol. 2011, article bar007.
Crosiers, D., J. Theuns, P. Cras, and C. Van Broeckhoven. 2011. Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. Journal of Chemical Neuroanatomy 42(2): 131–141.
Dunning, C.J., J.F. Reyes, J.A. Steiner, and P. Brundin. 2012. Can Parkinson’s disease pathology be propagated from one neuron to another? Progress in Neurobiology 97(2): 205–219.
Shulman, J.M., P.L. De Jager, and M.B. Feany. 2011. Parkinson’s disease: genetics and pathogenesis. Annual Review of Pathology 6: 193–222.
Obeso, J.A., M.C. Rodriguez-Oroz, C.G. Goetz, et al. 2010. Missing pieces in the Parkinson’s disease puzzle. Nature Medicine 16(6): 653–661.
Hirsch, E.C., and S. Hunot. 2009. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurology 8: 382–397.
Zhang, W., T. Wang, Z. Pei, D.S. Miller, X. Wu, M.L. Block, B. Wilson, W. Zhang, Y. Zhou, J.S. Hong, et al. 2005. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB Journal 19: 533–542.
Reynolds, A.D., I. Kadiu, S.K. Garg, J.G. Glanzer, T. Nordgren, P. Ciborowski, R. Banerjee, and H.E. Gendelman. 2008. Nitrated alpha-synuclein and microglial neuroregulatory activities. Journal of Neuroimmune Pharmacology 3: 59–74.
Chen, H., E.J. O’Reilly, M.A. Schwarzschild, and A. Ascherio. 2008. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. American Journal of Epidemiology 167: 90–95.
Wong, K.T., J.S. Grove, A. Grandinetti, J.D. Curb, M. Yee, P. Blanchette, et al. 2009. Association of fibrinogen with Parkinson disease in elderly Japanese-American men: a prospective study. Neuroepidemiology 34: 50–54.
Margis, R., and C.R. Rieder. 2011. Identification of blood microRNAs associated to Parkinson’s disease. Journal of Biotechnology 152: 96–101.
LeWitt, P. 2012. Recent advances in CSF biomarkers for Parkinson’s disease. Parkinsonism & Related Disorders 18(Suppl 1): 49–51.
LeWitt, P., L. Schultz, P. Auinger, and M. Lu. 2011. CSF xanthine, homovanillic acid, and their ratio as biomarkers of Parkinson’s disease. Brain Research 140: 88–97.
Orrell, R.W., J.J. Habgood, A. Malaspina, et al. 1999. Clinical characteristics of SOD1 gene mutations in UK families with ALS. Journal of Neurological Science 169: 56–60.
Blair, I.P., K.L. Williams, S.T. Warraich, et al. 2010. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. Journal of Neurology, Neurosurgery, and Psychiatry 81(6): 639–645.
Kabashi, E., P.N. Valdmanis, P. Dion, et al. 2008. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genetics 40: 572–574.
World Federation of Neurology Research Group on Neuromuscular Diseases Subcommittee on Motor Neuron Disease. Airlie House guidelines. 1995. Therapeutic trials in amyotrophic lateral sclerosis. Airlie House, “Therapeutic Trials in ALS,” Workshop Contributors. Journal of Neurological Science 129: 1–10.
Traynor, B.J., M.B. Codd, B. Corr, C. Forde, E. Frost, and O.M. Hardiman. 2000. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population-based study. Archives of Neurology 57: 1171–1176.
Liu, Y., W. Hao, A. Dawson, S. Liu, and K. Fassbender. 2009. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. Journal of Biological Chemistry 284: 3691–3699.
Yiangou, Y., P. Facer, P. Durrenberger, I.P. Chessell, A. Naylor, C. Bountra, R.R. Banati, and P.Y. Anand. 2006. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurology 6: 12.
Amit, I., M. Garber, N. Chevrier, A.P. Leite, Y. Donner, T. Eisenhaure, M. Guttman, J.K. Grenier, W. Li, O. Zuk, L.A. Schubert, B. Birditt, T. Shay, A. Goren, X. Zhang, Z. Smith, R. Deering, R.C. McDonald, M. Cabili, B.E. Bernstein, J.L. Rinn, A. Meissner, D.E. Root, N. Hacohen, and A. Regev. 2009. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326: 257–263.
Uranishi, H., T. Tetsuka, M. Yamashita, K. Asamitsu, M. Shimizu, M. Itoh, and T. Okamoto. 2001. Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-kappa B p65-mediated transcription as a coactivator. Journal of Biological Chemistry 276: 13395–13401.
McGeer, P.L., and E.G. McGeer. 2008. Glial reactions in Parkinson’s disease. Movement Disorders 23: 474–483.
Zhang, R., R. Gascon, R.G. Miller, et al. 2005. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). Journal of Neuroimmunology 159: 215–224.
Mantovani, S., S. Garbelli, A. Pasini, et al. 2009. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. Journal of Neuroimmunology 210: 73–79.
Keizman, D., O. Rogowski, S. Berliner, et al. 2009. Low-grade systemic inflammation in patients with amyotrophic lateral sclerosis. Acta Neurologica Scandinavica 119: 383–389.
Turner, M.R., M.C. Kiernan, P.N. Leigh, and K. Talbot. 2009. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurology 8: 94–109.
Nardo, G., S. Pozzi, M. Pignataro, E. Lauranzano, G. Spano, S. Garbelli, S. Mantovani, K. Marinou, L. Papetti, M. Monteforte, V. Torri, L. Paris, G. Bazzoni, C. Lunetta, M. Corbo, G. Mora, C. Bendotti, and V. Bonetto. 2011. Amyotrophic lateral sclerosis multiprotein biomarkers in peripheral blood mononuclear cells. PLoS One 6(10): e25545.
Lu, C.H., A. Petzold, B. Kalmar, J. Dick, A. Malaspina, and L. Greensmith. 2012. Plasma neurofilament heavy chain levels correlate to markers of late stage disease progression and treatment response in SOD1(G93A) mice that model ALS. PLoS One 7(7): e40998. doi:10.1371/journal.pone.0040998.
Mitchell, R.M., W.M. Freeman, W.T. Randazzo, H.E. Stephens, J.L. Beard, Z. Simmons, et al. 2009. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 72: 14–19.
Beuche, W., M. Yushchenko, M. Mader, M. Maliszewska, K. Felgenhauer, and F. Weber. 2000. Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport 11: 3419–3422.
Walker, F.O. 2007. Huntington’s disease. Lancet 369(9557): 218–228.
Moller, T. 2010. Neuroinflammation in Huntington’s disease. Journal of Neural Transmission 117(8): 1001–1008.
Li, S.H., and X.J. Li. 2004. Huntingtin–protein interactions and the pathogenesis of Huntington disease. Trends in Genetics 20: 146–154.
Orr, H.T., and H.Y. Zoghbi. 2007. Trinucleotide repeat disorders. Annual Review of Neuroscience 30: 575–621.
Imarisio, S., et al. 2008. Huntington disease: from pathology and genetics to potential therapies. Biochemistry Journal 412: 141–209.
Roze, E., et al. 2008. Pathophysiology of Huntington’s disease: from huntingtin functions to potential treatments. Current Opinion in Neurology 21: 495–503.
Björkqvist, M., E.J. Wild, J. Thiele, A. Silvestroni, R. Andre, N. Lahiri, et al. 2008. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. Journal of Experimental Medicine 205: 1869–1877.
Khoshnan, A., J. Ko, E.E. Watkin, L.A. Paige, P.H. Reinhart, and P.H. Patterson. 2004. Activation of the IkappaB kinase complex and nuclear factor-kappaB contributes to mutant huntingtin neurotoxicity. Journal of Neuroscience 24: 7999–8008.
Cho, I.H., J. Hong, E.C. Suh, J.H. Kim, H. Lee, J.E. Lee, et al. 2008. Role of microglial IKK beta in kainic acid-induced hippocampal neuronal cell death. Brain 131: 3019–3303.
Kurlan, R., E. Caine, A. Rubin, et al. 1988. Cerebrospinal fluid correlates of depression in Huntington’s disease. Archives of Neurology 45: 881–883.
Schwarcz, R., C.A. Tamminga, R. Kurlan, et al. 1988. Cerebrospinal fluid levels of quinolinic acid in Huntington’s disease and schizophrenia. Annals of Neurology 24: 580–582.
Montine, T.J., M.F. Beal, D. Robertson, et al. 1999. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology 52: 1104–1105.
Jeitner, T.M., M.B. Bogdanov, W.R. Matson, et al\. 2001. N(epsilon)-(gamma-L-glutamyl)-L-lysine (GGEL) is increased in cerebrospinal fluid of patients with Huntington’s disease. Journal of Neurochemistry 79: 1109–1112.
Acknowledgments
This work was partially supported by the Italian Ministry of Economy and Finance with the “PNR-CNR Aging Program 2012-2014” project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Domenico Nuzzo and Pasquale Picone contributed equally to this article.
Rights and permissions
About this article
Cite this article
Nuzzo, D., Picone, P., Caruana, L. et al. Inflammatory Mediators as Biomarkers in Brain Disorders. Inflammation 37, 639–648 (2014). https://doi.org/10.1007/s10753-013-9780-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9780-2