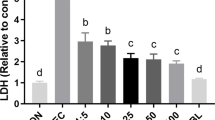
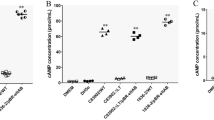
Abstract
Enteropathogenic Escherichia coli (EPEC) infects the human intestinal epithelium and is a major cause of infantile diarrhea in developing countries. Nitric oxide (NO) is an important modulator of intestinal inflammatory response. The aim of the present study was to investigate whether EPEC outer membrane proteins (OMPs) up regulate epithelial cell expression of inducible nitric oxide synthase (iNOS) and to examine the role of NF-κB and MAP kinases (MAPK) on nitrite production. iNOS mRNA expression was assessed by RT-PCR. Nitrite levels were measured by Griess reaction. NF-κB activation by OMPs was evaluated by EMSA and immunoblotting was done to detect MAPK activation. EPEC OMP up regulated iNOS, induced nitrite production and NF-κB and MAPK were activated in caco-2 cells. The nitrite levels decreased when NF-κB and MAPK inhibitors were used. Thus, EPEC OMPs induce iNOS expression and NO production through activation of NF-κB and MAPK.
Similar content being viewed by others
References
Levine, M. M., and R. Edelman. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: Epidemiology and pathogenesis. Epidemiol. Rev. 6:31–51.
Kenny, B., R. DeVinney, M. Stein, D. Reinscheid, E. Frey, and B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520.
Savkovic, S., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160–C1167.
Savkovic, S., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480–4487.
Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298–1305.
de Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217–6224.
Hecht, G. 2001. Microbes and microbial toxins: Paradigms for microbial mucosal interactions. VII. Enteropathogenic Escherichia coli: Physiological alterations from an extracellular position. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1–G7.
Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G890–G898.
Baldwin, T. J., S. F. Brooks, S. Knutton, H. A. Manjarrez Hernandez, A. Aitken, and P. H. Williams. 1990. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect. Immun. 58:761–765.
Rosenshine, I., M. Donnenberg, J. B. Kaper, and B. B. Finlay. 1992. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 11:3551–3560.
Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI3-kinase-dependent pathways. EMBO J. 20:1245–1258.
Witthoft, T., L. Eckmann, J. M. Kim, and M. F. Kagnoff. 1998. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. 275:G564–G571.
Vallance, B. A., W. Deng, M. D. Grado, C. Chan, K. Jacobson, and B. B. Finlay. 2002. Modulation of inducible nitric oxide synthase expression by attaching and effacing bacterial pathogen Citrobacter rodentium in infected mice. Infect. Immun. 70:6424–6435.
Davis, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553–14556.
Zhou, X., A. J. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120–2129.
Xie, Q.-W., Y. Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269:4705–4708.
Goldring, C. E. P., R. Narayanan, P. Lagadec, and J.-F. Jeannin. 1995. Transcriptional inhibition of the inducible nitric oxide synthase gene by competitive binding of NF-κB/Rel proteins. Biochem. Biophys. Res. Commun. 209:73–79.
Kumar, S. S., V. Malladi, K. Sankaran, R. Haigh, P. H. Williams, and A. Balakrishnan. 2001. Extrusion of actin-positive strands from HEp-2 and Int 407 cells caused by outer membrane preparations of enteropathogenic Escherichia coil and specific attachment of wild type bacteria to the Strands. Can. J. Microbiol. 47:727–734.
Kumar, S. S., K. Sankaran, R. Haigh, P. H. Williams, and A. Balakrishnan. 2001. Cytopathic effects of outer-membrane preparations of enteropathogenic Escherichia coli and co-expression of maltoporin with secretory virulence factor, EspB. J. Med. Microbiol. 50:602–612.
Malladi, V., B. Shankar, P. H. Williams, and A. Balakrishnan. 2004. Enteropathogenic Escherichia coli outer membrane proteins induce changes in cadherin junctions of caco-2 cells through activation of PKCα. Microbes Infect. 6:38–50.
Finlay, B. B., I. Rosenshine, M. S. Donnenberg, and J. B. Kaper. 1992. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect. Immun. 60:2541–2543.
Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: Basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290–1298.
Baldini, M. M., J. B. Kaper, M. M. Levine, D. C. Candy, and H. W. Moon. 1983. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J. Pediatr. Gastroenterol. Nutr. 2:534–538.
Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317.
Jerse, A. E., K. G. Gicquelais, and J. B. Kaper. 1991. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect. Immun. 59:3869–3875.
Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302–4309.
Miyamoto, S., P. J. Chiao, and I. M. Verma. 1994. Enhanced IκBα degradation is responsible for constitutive NF-κB activity in mature murine B-cell lines. Mol. Cell. Biol. 14:3276–3282.
Narayanan, K., A. Balakrishnan, and S. Miyamoto. 2000. NF-κB is essential for induction of proinflammatory cytokine genes by filarial parasitic sheath proteins. Mol. Immunol. 37:115–123.
Hall, L. R., R. K. Mehlotra, A. W. Higgins, M. A. Haxhiu, and E. Pearlman. 1998. An essential role for interleukin-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect. Immun. 66:4425–4430.
Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 126:131–138.
Savkovic, S. D., A. Koutsouris, and G. Hecht. 2003. PKC ζ participates in activation of inflammatory response induced by enteropathogenic E. coli. Am. J. Physiol. Cell Physiol. 285:C512–C521.
Foubister, V., I. Rosenshine, and B. B. Finlay. 1994. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J. Exp. Med. 179:993–998.
Kim, J. M., J. S. Kim, H. C. Jung, I. S. Song, and C. Y. Kim. 2002. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori-infected human gastric epithelial cells: Possible role of interferon-γ in polarized nitric oxide secretion. Helicobacter 7:116–125.
Mysorekar, I. U., M. A. Mulvey, S. J. Hultgren, and J. I. Gordon. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412–7419.
Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, and S. G. Kachalsky. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to betal integrins. J. Biol. Chem. 271:20359–20364.
Frankel, G., A. D. Phillips, L. R. Trabulsi, S. Knutton, G. Dougan, and S. Matthews. 2001. Intimin and the host cell—Is it bound to end in Tir(s)?. Trends. Microbiol. 9:214–218.
Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588–591.
Didierlaurent, A., J. C. Sirard, J. P. Kraehenbuhl, and M. R. Neutra. 2002. How the gut senses its content. Cell. Microbiol. 4:61–72.
Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361–379.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Malladi, V., Puthenedam, M., Williams, P.H. et al. Enteropathogenic Escherichia coli Outer Membrane Proteins Induce iNOS by Activation of NF-κB and MAP Kinases. Inflammation 28, 345–353 (2004). https://doi.org/10.1007/s10753-004-6645-8
Issue Date:
DOI: https://doi.org/10.1007/s10753-004-6645-8