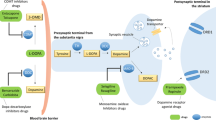
Abstract
The review considers the main molecular physiological causes of neurodegenerative disorders. The genetic factors involved in Parkinson’s and Alzheimer’s diseases are conventionally divided into pharmacodynamic and pharmacokinetic. The former are analyzed at the levels of dopamine (DA) neurons and polymorphism of the D1, D2, and D3 DA receptors. The role of polymorphisms of some proteins such as parkin (PARK1-PARK10) and α-synuclein in generation of Lewy bodies is described. The pharmacokinetic factors play a role in Parkinson’s disease (PD) at the level of metabolism of DA, dioxyphenylalanine, and tyrosine and include polymorphisms of enzymes and proteins involved in the relevant metabolic reactions. The profile of DA metabolites may contribute to neurotoxicity and the development of PD. Prospects of drug therapy of PD and the risk of adverse drug effects such as mental disorders and dyskinesia are considered in terms of polymorphisms of enzymes and transport proteins.
Similar content being viewed by others
REFERENCES
Pharmacogenomics: The Search for Individualized Therapies, Licinio, J. and Wong, M., Eds., Weinheim: Wiley-VCH, 2002.
Piruzyan, L.A., On Pharmacological Metrology, Izv. Akad. Nauk SSSR, Ser. Biol., 1990, no. 2, p. 302.
Piruzyan, L.A., Sukhanov, V.A., and Saprin, A.N., Factor Predicting the Risk of Pathological Processes from Polymorphisms of Xenobiotic-Metabolizing Enzymes, Fiziol. Chel., 2000, vol. 26, no.2, p. 115.
Piruzyan, L.A., Sukhanov, V.A., Kalinina, E.V., and Saprin, A.N., Medicobiological Aspects of Metabolic Portraying, Dokl. Akad. Nauk, 2001, vol. 377, p. 129.
Piruzyan, L.A., Sukhanov, V.A., Kalinina, E.V., et al., Enzymatic System of Xenobiotic Metabolism and Detoxification As a Basis for Metabolic Portraying and Predicting the Risk of Pathological Processes, Izv. Akad. Nauk, Ser. Biol., 2002, no. 2, p. 149.
Piruzyan, L.A. and Mikhailovskii, E.M., Metabolic “In Vivo Designing” of Tumors at the Levels of an Organism and Population under Conditions of Individual Genetic Predisposition: Communication I, Fiziol. Chel., 2001, vol. 27, no.3, p. 113.
Piruzyan, L.A. and Mikhailovskii, E.M., Metabolic “In Vivo Designing” of Tumors at the Levels of an Organism and Population under Conditions of Individual Genetic Predisposition: Communication II, Fiziol. Chel., 2002, vol. 28, no.1, p. 101.
Piruzyan, L.A. and Mikhailovskii, E.M., Metabolic “In Vivo Designing” of Tumors at the Levels of an Organism and Population under Conditions of Individual Genetic Predisposition: Communication III, Fiziol. Chel., 2002, vol. 28, no.5, p. 103.
Piruzyan, L.A. and Mikhailovskii, E.M., Metabolic “In Vivo Designing” of Tumors at the Levels of an Organism and Population under Conditions of Individual Genetic Predisposition: Communication IV, Fiziol. Chel., 2003, vol. 29, no.2, p. 118.
Piruzyan, L.A., Radkevich, L.A., and Morozova, N.V., Ethnic Metabolic Portraying in Selecting Drug Therapy with the Example of N-Acetylation and Chronic Diseases of the Liver, Dokl. Akad. Nauk, 2003, vol. 388, p. 842.
Jellinger, K.A., Cell Death Mechanisms in Neurodegeneration, J. Cell. Mol. Med., 2001, vol. 5, p. 1.
Jellinger, K.A. and Bancher, C., Neuropathology of Alzheimer’s Disease: A Critical Update, J. Neural. Transm. (Suppl.), 1998, vol. 54, p. 77.
Jelinger, K.A., Neuropathology of Movement Disorders, Neurosurg. Clin. North. Am., 1998, vol. 9, p. 237.
McKeith, I.G., Galasko, D., Kosaka, K., et al., Consensus Guidelines for the Clinical and Pathological Diagnosis of Dementia with Lewy Bodies, Neurology, 1996, vol. 47, p. 1113.
Julien, J.P. and Beaulieu, J.M., Cytoskeletal Abnormalities in Amyotrophic Lateral Sclerosis, J. Neurol. Sci., 2000, vol. 180, p. 7.
Saunders, C.D., Parkinson’s Disease: A New Hope, Boston: Harvard Health, 2000.
Kamenetskii, V.K., Parkinsonizm (Parkinsonism), St. Petersburg: Piter, 2001.
Kitamura, Y., Kakimura, J., and Taniguchi, T., Antiparkinsonian Drugs and Their Neuroprotective Effects, Biol. Pharm. Bull., 2002, vol. 25, p. 284.
Mel’nichuk, P.V. and Voevodskaya, I.V., Parkinsonizm. Istoriya, etiologiya i klinika (Parkinsonism: History, Etiology and Clinics), Moscow: Meditsina, 1974.
Golubev, V.L., Levin, Ya.I., and Vein, A.M., Bolezn’ Parkinsona i sindrom Parkinsona (Parkinson Disease and Parkinsonism), Moscow: Medpress, 2000.
Dekker, M.C., Bonifati, V., and van Duijn, C.M., Parkinson Disease: Piecing Together a Genetic Jigsaw, Brain, 2003, vol. 126, p. 1722.
Kurland, L.T., Kurtzke, J.F., and Goldberg, I.D., Epidemiology of Neurological and Sense Organ Disorders, Cambridge, 1973.
Paddison, R. and Gnittith, R. Occurrence of Parkinson’s Disease in Black Patients at Charity Hospital in New Orleans, Neurology, 1974, vol. 24, p. 688.
Jellinger, K., Pathology of Parkinson, Fahn, S., Ed., New York: Raven, 1986.
Lieberman, A.N. and Shupak, J.L., Levodopa and Melanoma, Neurology, 1974, vol. 31, p. 188.
De Jong, D., Parkinson Disease: Statistics, J. Neurosurg., 1966, vol. 24.suppl, p. 149.
Lu, A.Y., Drug-Metabolism Research Challenges in the New Millennium: Individual Variability in Drug Therapy and Drug Safety, Drug Metab. Dispos., 1998, vol. 26, p. 1217.
Kitamura, Y., Kakimura, J., and Taniguchi, T., Antiparkinsonian Drugs and Their Neuroprotective Effects, Biol. Pharm. Bull., 2002, vol. 25, p. 284.
Stout, J.G., Zhou, Q., Wiedmer, T., and Sims, P.J., Glutamate Receptors and Calcium, Biochemistry, 1998, vol. 37, p. 14 860.
Polimeropoulos, M.H., Higgins, J.J., Golbe, L.I., et al., Mapping of a Gene for Parkinson’s Disease to Chromosome 4q21–q23, Science, 1996, vol. 274, p. 1197.
Clayton, D.F. and George, J.M., Synucleins in Synaptic Plasticity and Neurodegenerative Disorders, J. Neurosci. Res., 1999, vol. 58, p. 120.
Conway, D.F., Harper, J.M., and Lansbury, P.T., Accelerated In Vitro Fibril Formation by Mutant α-Synuclein Linked to Early-Onset Parkinson’s Disease, Nat. Med., 1998, vol. 4, p. 1318.
Lin, S.K., Lu, L.S., and Vingerhoets, B.J., Isolated Involvement of Substantia Nigra, Parkinsonism Relat. Disord., 1995, vol. 3, p. 67.
Zarranz, J.J., Alegre, J., Gomez-Esteban, A., et al., The New Mutation, E46K, of α-Synuclein Causes Parkinson and Lewy Body Dementia, Ann. Neurol., 2004, vol. 55, p. 153.
Fornai, F., Lenzi, P., Gesi, M., et al., Resent Knowledge on Molecular Components of Lewy Bodies, Curr. Drug Target CNS Neurol. Disord., 2003, vol. 2, p. 149.
Shastry, B., Neurodegenerative Disorders of Protein Aggregation, Neurochem. Internat., 2003, vol. 43, p. 1.
Huynh, D.P., Scoles, D.R., Nguyen, D., and Pulst, S.M., The Autosomal Recessive Juvenile Parkinson Disease Gene Product, Parkin, Interacts with and Ubiquitinates Synaptotagmin XI, Hum. Mol. Genet., 2003, vol. 12, p. 2587.
Baptista, M.J., Cookson, M.R., and Miller, D.W., Parkin and α-Synuclein: Opponent Actions in the Pathogenesis of Parkinson Disease, Neuroscientist, 2004, vol. 10, p. 63.
Mori, H., Kond, O.T., Yokochi, M., et al., Pathologic and Biochemical Studies of Juvenile Parkinsonism Linked to Chromosome 6q, Neurology, 1998, vol. 51, p. 890.
Satoh, J. and Kuroda, Y., Association of Codon 167 Ser/Asn Heterozigosity in the Parkin Gene with Sporadic Parkinson’s Disease, NeuroReport, 1999, vol. 10, p. 2735.
Wang, M., Hattori, N., Matsumine, H., et al., Polymorphism in the Parkin Gene in Sporadic Parkinson’s Disease, Ann. Neurol., 1999, vol. 45, p. 655.
Lucking, C.B., Chesneau, V., Logmann, E., et al., Coding Polymorphisms in the Parkin Gene and Susceptibility to Parkinson Disease, Arch. Neurol., 2003, vol. 60, p. 1253.
Denison, S.R., Callahan, G., Becker, N.A., Characterization of FRA6E and Its Potential Role in Autosomal Recessive Juvenile Parkinsonism and Ovarian Cancer, Genes Chromosomes Cancer, 2003, vol. 38, p. 40.
Hedrich, K., Djarmati, A., Schafer, N., et al., DJ-1 (PARK7) Mutations Are Less Frequent Than Parkin (PARK2) Mutations in Early-Onset Parkinson Disease, Neurology, 2004, vol. 62, p. 389.
Karamohamed, S., DeStefano, A.L., Wilk, J.B., et al., A Haplotype at the PARK3 Locus Influences Onset Age for Parkinson’s Disease, Neurology, 2003, vol. 61, p. 1557.
Healy, D.G., Abou-Sleiman, P.M., and Valente, E.M., DJ-1 Mutations in Parkinson’s Disease, J. Neurol. Neurosurg. Psychiatry, 2004, vol. 75, p. 144.
Gorner, K., Holtorf, E., Odoy, S., et al., Differential Effects of Parkinson’s Disease-Associated Mutations on Stability and Folding of DJ-1, J. Biol. Chem., 2004, vol. 279, p. 6943.
Miller, D.W., Ahmad, R., Hague, S., et al., L166P Mutant DJ-1, Causative for Recessive Parkinson’s Disease, Is Degraded through the Ubiquitin-Proteasome System, J. Biol. Chem., 2003, vol. 278, p. 36 588.
Morris, C.M., O’Brien, K.K., Gibson, A.M., et al., Polymorphism in the Human DJ-1 Gene Is Not Associated with Sporadic Dementia with Lewy Bodies or Parkinson’s Disease, Neurosci. Lett., 2003, vol. 352, p. 151.
Bandopadhyay, R., Kihgsbury, A.E., Cookson, M.R., et al., The Expression of DJ-1 (PARK7) in Normal Human CNS and Idiopathic Parkinson’s Disease, Brain, 2004, vol. 127, p. 420.
Leroy, E., Boyer, R., Auburger, G., et al., The Ubiquitin Pathway in Parkinson’s Disease, Nature, 1998, vol. 395, p. 451.
Maraganore, D.M., Farrer, M.J., and Hardy, J.A., Case Control Study of the Ubiquitin Carboxy-Terminal Hydrolase L1 Gene in Parkinson’s Disease, Neurology, 1999, vol. 53, p. 1858.
Maimone, D., Dominici, R., and Grimaldi, L., Pharmacogenomics of Neurodegenerative Diseases, Eur. J. Pharmacol., 2001, vol. 413, p. 11.
Cookson, M., Crystallizing Ideas about Parkinson’s Disease, Proc. Natl. Acad. Sci. USA, 2003, vol. 100, p. 9111.
Schulte, T., Bohringer, S., Schols, L., et al., Modulation of Disease Risk According to a Cathepsin D/Apolipoprotein E Genotype in PD, J. Neuronal. Transm., 2003, vol. 110, p. 749.
Zimprich, A., Muller-Myhsok, B., Farrer, M., et al., The PARK8 Locus in Autosomal Dominant Parkinsonism, Am. J. Hum. Genet., 2004, vol. 74, p. 11.
Hicks, A.A., Petursson, H., Jonsson, T., et al., A Susceptibility Gene for Late-Onset Idiopathic Parkinson’s Disease, Ann. Neurol., 2002, vol. 52, p. 549.
Wirdefeldt, K., Burgess, C.E., Westerberg, L., and Schalling, M., A Linkage Study of Candidate Loci in Familial Parkinson’s Disease, Am. J. Hum. Genet., 2003, vol. 69, p. 24.
Djarmati, A., Hedrich, K., Svetel, M., et al., Detection of Parkin (PARK2) and DJ1 (PARK7) Mutations in Early-Onset Parkinson Disease, Hum. Mutat., 2004, vol. 23, p. 525.
Eerola, J., Hernandez, D., and Launes, J., Assessment of DJ1 (PARK7) Polymorphism in Finnish PD, Neurology, 2003, vol. 61, p. 1000.
Oliveira, S.A., Scott, W.K., Nance, M.A., et al., Association Study of Parkin Gene Polymorphisms with Idiopathic Parkinson Disease, Arch. Neurol., 2003, vol. 60, p. 975.
Benbunan, B.R., Korczyn, A.D., and Giladi, N., Parkin Mutation-Associated Parkinsonism and Cognitive Decline, Comparison to Early-Onset Parkinson’s Disease, J. Neuronal. Transm., 2004, vol. 111, p. 47.
West, A.B. and Maidment, N.T., Genetics of Parkin-Linked Disease, Hum. Genet., 2004, vol. 114, p. 327.
Carr, J., Fuente, M., Schulzer, E., et al., Familial and Sporadic Parkinson’s Disease Usually Display the Same Clinical Features, Parkinsonism Related Disord., 2003, vol. 9, p. 201.
Lincoln, S.J., Maraganore, D.M., Lesnick, T.J., et al., Parkin Variants in North American Parkinson’s Disease: Cases and Controls, Mov. Disord., 2003, vol. 18, p. 1306.
Maher, N.E., Golbe, L.I., Lazzarini, A.M., et al., Epidemiologic Study of 203 Sibling Pairs with Parkinson’s Disease: The Gene PD Study, Neurology, 2002, vol. 58, p. 79.
Berger, K., Breteler, M.M., Helmer, C., et al., Prognosis with Parkinson’s Disease in Europe, Neurology, 2000, vol. 54,suppl. 5, p. S24.
Smargiassi, A., Mutti, A., De Rossa, A., et al., A Case-Cotrol Study of Occupational and Environmental Risk Factors for Parkinson’s Disease, Neurotoxicology, 1998, vol. 19, p. 709.
Pigullo S., Di Maria E., Marchese R., et al., Essential Tremor Is Not Associated with Synuclein Gene Haplotypes, Mov. Disord., 2003, vol. 18, p. 823.
Goider-Khouja, N., Belal, S., Hamida, M.B., and Hentati, F., Clinical and Genetic Study of Familial Parkinson’s Disease in Tunisia, Neurology, 2000, vol. 54, p. 1603.
Behari, M., Strivastava, A.K., Das, R.R., and Pandey, R.M., Risk Factors of Parkinson’s Disease in Indian Patients, J. Neurol. Sci., 2001, vol. 190, p. 49.
Kruger, R., Fischer, C., Schulte, T., et al., Mutation Analysis of the Neurofilament M Gene in Parkinson’s Disease, Neurosci. Lett., 2003, vol. 351, p. 125.
Beal, M.F., Bioenergetic Approaches for Neuroprotection in Parkinson’s Disease, Ann. Neurol., 2003, vol. 53,suppl. 3, p. S39.
Ebadi, M. and Sharma, S.K., Peroxynitrite and Mitochondrial Dysfunction in the Pathogenesis of Parkinson’s Disease, Antioxid. Redox Signal., 2003, vol. 5, p. 319.
Mellick, G.D., Silburn, P.A., Prince, J.A., and Brookes, A.J., A Novel Screen for Nuclear Mitochondrial Gene Associations with Parkinson’s Disease, J. Neuronal. Transm., 2004, vol. 111, p. 191.
Dekker, M.C., Giesbergen, P.C., Njajou, O.T., et al., Mutations in the Hemochromatosis gene (HFE), Parkinson’s Disease and Parkinsonism, Neurosci. Lett., 2003, vol. 348, p. 117.
Felletschin, B., Bauer, P., Walter, U., et al., Screening for Mutations of the Ferritin Light and Heavy Genes in Parkinson’s Disease with Hyperechogenicity of the Substantia Nigra, Neurosci. Lett., 2003, vol. 352, p. 53.
Froelich, S., Axelman, P., Almkvist, A., et al., Extended Investigation of τ and Mutation Screening of Other Candidate Genes on Chromosome 17q21 in Swedish FTDP-17 Family, Am. J. Med. Genet. B, 2003, vol. 121, p. 112.
Clark, L.N., Levy, G., Tang, M.X., et al., The Saitohin ‘Q7R’ Polymorphism and τ Haplotype in Multi-Ethnic Alzheimer Disease and Parkinson’s Disease Cohort, Neurosci. Lett., 2003, vol. 347, p. 17.
Wu, Y.R., Wang, C.K., Chen, C.M., et al., Analysis of Heat-Shock Protein 70 Gene Polymorphisms and the Risk of Parkinson’s Disease, Hum. Genet., 2004, vol. 114, p. 236.
Carminee, A., Buervenich, S., Galter, D., et al., NUUR1 Promoter Polymorphisms: Parkinson’s Disease, Schizophrenia, and Personality Traits, Am. J. Med. Genet. B, 2003, vol. 120, p. 51.
Van Der Walt, D., Noureddine, M.A., Kittappa, R., et al., Fibroblast Growth Factor 20 Polymorphisms and Haplotypes Strongly Influence Risk of Parkinson Disease, Am. J. Hum. Genet., 2004, vol. 74, p. 1121.
Lwin, A., Orvisky, E., Goker-Alpan, O., et al., Glucocerebrosidase Mutations in Subjects with Parkinsonism, Mol. Genet. Metab., 2004, vol. 81, p. 70.
Smith, P., Crocker, S., Jackson, V., and Park, D., Cyclin-Cependent Kinase 5 Is a Mediator of Dopaminergic Neuron Loss, Proc. Natl. Acad. Sci. USA, 2003, vol. 100, p. 13 650.
Wang, J., Si, J.M., Liu, Z.L., and Yu, L., Cholecystokinin, A and B-Receptor Gene Polymorphisms in Parkinson’s Disease, Pharmacogenetics, 2003, vol. 13, p. 365.
Bresman, S.B., Dystonia: Phenotypes and Genotypes, Rev. Neurol., 2003, vol. 159, p. 849.
Kabakci, K., Hedrich, K., Leung, J.C., et al., Mutations in DYT1: Extension of the Phenotypic and Mutational Spectrum, Neurology, 2004, vol. 62, p. 395.
Tan, L.C., Tanner, C.M., Chen, R., et al., Marked Variation in Clinical Presentation and Age of Onset in Family with a Heterozygous Parkin Mutation, Mov. Disord., 2003, vol. 18, p. 758.
Lampe, J.B., Gossrau, G., Herting, B., et al., HLA Typing and Parkinson Disease, Eur. Neurol., 2003, vol. 50, p. 64.
Czlonkowska, A., Kurkowska, I., Czlonkowski, A., et al., Immune Processes in the Pathogenesis of Parkinson’s Disease—A Potential Role for Microglia and Nitric Oxide, Med. Sci. Monit., 2002, vol. 8, p. 165.
Imamura, K., Hishikawa, N., Sawada, M., et al., Distribution of Major Histocompatibility Complex Class II-Positive Microglia, Acta Neuropathol., 2003, vol. 106, p. 518.
Daveu, C., Servy, C., Dendane, M., et al., Oxidation and Nitration of Catecholamines by Nitrogen Oxides Derived from Nitric Oxide, Nitric Oxide, 1997, vol. 3, p. 234.
Foppoli, C., Coccia, R., Cini, C., and Rosei, M., Catecholamines Oxidation by Xanthine Oxidase, Biochim. Biophys. Acta, 1997, vol. 1334, p. 200.
Graham, D.G., Oxidative Pathways for Catecholamines in the Genesis of Neuromelanin and Cytotoxic Quinones, Mol. Pharmacol., 1978, vol. 14, p. 633.
Hastings, T.G., Enzymatic Oxidation of Dopamine, J. Neurochem., 1995, vol. 64, p. 919.
Hawley, M.D., Tatawawadi, S.V., and Piekarsky, S., Electrochemical Studies of the Oxidation Pathways of Cathecholamines, J. Am. Chem. Soc., 1967, vol. 89, p. 447.
Kostrzewa, R.M. and Segura-Aguilar, J., Neurotoxicological and Neuroprotective Elements in Parkinson’s Disease, Neurotox. Res., 2002, vol. 4, p. 83.
Segura-Aguilar, J., Peroxidase Activity of Liver Microsomal Vitamin D 25 Hydroxylase Catalyzes 25-Hydroxylation of Vitamine D3 and Oxidation of Dopamine to Aminochrome, Biochem. Mol. Med., 1996, vol. 58, p. 122.
Segura-Aguilar, J. and Lind, C., On the Mechanism of the Mn3+-Induced Neurotoxicity of Dopamine, Chem. Biol. Interact., 1989, vol. 72, p. 309.
Teriand, O., Flatmark, T., Tangeras, A., and Gronberg, M., Dopamine Oxidation Generates an Oxidative Stress Mediated by Dopamine Semiquinone and Unrelated to Reactive Oxygen Species, J. Mol. Cell Cardiol., 1997, vol. 29, p. 1731.
Shen, X.M., Xia, B., Wrona, M.Z., and Dryhurst, G., Synthesis, Redox Properties, In Vivo Formation, and Neurobehavioral Effects of N-Acetylcysteinyl Conjugates of Dopamines, Chem. Res. Toxicol., 1996, vol. 9, p. 1117.
Healy, D.G., Abou-Sleiman, P.M., Ozawa, T., et al., A Functional Polymorphism Regulating Dopamine β-Hydroxylase Influences against Parkinson’s Disease, Ann. Neurol., 2004, vol. 55, p. 443.
Tan, E.K., Chai, A., Lum, S.Y., et al., Monoamine Oxidase B Polymorphism, Am. J. Med. Genet. B, 2003, vol. 120, p. 58.
McLeod, H.L., Syvanen, A.C., Githang, J., et al., Ethnic Differences in Catechol O-Methyltransferase Pharmacogenetics, Pharmacogenetics. 1998, vol. 8, p. 195.
Hoda, F., Nicholl, D., Bennett, P., et al., No Association between Parkinson Disease and Low-Activity Alleles of COMT, Biochem. Biophys. Res. Commun., 1996, vol. 228, p. 780.
Syvanen, A.C., Tilgman, C., Rinne, G., et al., Genetic Polymorphism of COMT, Pharmacogenetics, 1997, vol. 7, p. 65.
Xie, T., Ho, S.L., Li, L.S., and Mao, O.C., G/A1947 Polymorphism in COMT Gene in Parkinson’s Disease, Mov. Disord., 1997, vol. 12, p. 426.
Yoritaka, A., Hattori, N., Yoshino, H., and Mizuno, Y., COMT Genotype and Susceptibility to Parkinson’s Disease in Japan, J. Neuronal. Transm., 1997, vol. 104, p. 1313.
Wu, R.M., Cheng, S.W., Chen, K.H., et al., The COMT L Allele Modifies the Association between MAOB Polymorphism and PD in Taiwanese, Neurology, 2001, vol. 56, p. 375.
Watanabe, M., Harada, S., Nakamura, T., et al., Association between COMT Gene Polymorphisms and Dyskinesia in Parkinson’s Disease, Neuropsychobiology, 2003, vol. 48, p. 190.
Li, Y.J., Oliveira, S.A., Xu, P., et al., Glutathione S-Transferase ω-1 Modifies Age-at-Onset of Alzheimer Disease and Parkinson Disease, Hum. Mol. Genet., 2003, vol. 12, p. 3259.
Harhangi, B.S., Oostra, B.A., Heutink, P., et al., NAT-2 Polymorphism in Parkinson’s Disease, J. Neurol. Neurosurg. Psychiatry, 1999, vol. 67, p. 518.
Kaiser, R., Hofer, A., Grapengiesser, A., et al., L-Dopa-Induced Adverse Effects in PD and Dopamine Transporter Gene Polymorphism, Neurology, 2003, vol. 60, p. 1750.
Makoff, A.J., Graham, J.M., Arranz, M.J., et al., Association Study of Dopamine Receptor Gene Polymorphisms with Drug-Induced Hallucinations in Patients with Idiopathic Parkinson’s Disease, Pharmacogenetics, 2000, vol. 10, p. 43.
Wang, J., Zhao, C., Chen, B., and Liu, Z.L., Polymorphisms of Dopamine Receptor and Transporter Gene Hallucinations in Parkinson’s Disease, Neurosci. Lett., 2004, vol. 355, p. 193.
Oliveri, R.L., Annesi, G., Zappia, M., et al., Dopamine D2 Receptor Gene Polymorphism and the Risk of Levodopa-Induced Dyskinesias in PD, Neurology, 1999, vol. 53, p. 1425.
Nakasoa, K., Yasuia, K., Kowaa, H., et al., Hypertrophy of IMC of Carotid Artery in Parkinson’s Disease Is Associated with L-DOPA, Homocysteine, and MTHFR Genotype, J. Neurol. Sci., 2003, vol. 207, p. 19.
Youdim, M.B. and Weinstock, M., Molecular Basis of Neuroprotective Activities of Rasagiline and the Anti-Alzheimer Drug, Cell Mol. Neurobiol., 2001, vol. 21, p. 555.
Mori, S., Responses to Donepezil in Alzheimer’s Disease and Parkinson’s Disease, Ann. N. Y. Acad. Sci., 2002, vol. 977, p. 493.
Saporito, M.S., Hudkins, R.L., and Maroney, A.C., Discovery of CEP-1347/KT-7515, an Inhibitor of the JNK/SAPK Pathway for the Treatment of Neurodegenerative Diseases, Prog. Med. Chem., 2002, vol. 40, p. 23.
Pisani, A., Bonsi, P., Centonze, D., et al., Targeting Striatal Cholinergic Interneurons in Parkinson’s Disease, Neuropharmacology, 2003, vol. 45, p. 45.
Storch, A., Hwang, Y.I., Gearhart, D.A., and Beach, J.W., Dopamine Transporter-Mediated Cytotoxicity of β-Carbolinium Derivatives Related to Parkinson’s Disease: Relationship to Transporter-Dependent Uptake, J. Neurochem. 2004, vol. 89, p. 685.
Jost, W.H. and Bruck, C., Drug Interactions in the Treatment of Parkinson’s Disease, J. Neurol., 2002, vol. 249,suppl. 3, p. III/24.
Muller, T., Drug Treatment of Non-Motor Symptoms in Parkinson’s Disease, Expert Opin. Pharmacother., 2002, vol. 3, p. 381.
Gimenez-Roldan, S., Navarro, E., and Mateo, D., Effects of Quetiapine at Low Doses on Psyhosis, Motor Disability and Stress of the Caregiver in Patients with Parkinson’s Disease, Rev. Neurol., 2003, vol. 36, p. 401.
Tariot, P.N. and Ismail, M.S., Use of Quetiapine in Elderly Patients, J. Clin. Psychiatry, 2002, vol. 63,suppl. 13, p. 21.
Elbaz, A., Levecque, C., Clavel, J., et al., CYP2D6 Polymorphism, Pesticide Exposure, and Parkinson’s Disease, Ann. Neurol., 2004, vol. 55, p. 430.
Persad, A.S., Stedeford, T., Tanaka, S., et al., Parkinson’s Disease and CYP2D6 Polymorphism in Asian Populations, Neuroepidemiology, 2003, vol. 22, p. 357.
Weisgraber, K.H. and Mahley, R.W., Human Apolipoprotein E: The Alzheimer’s Disease Connection, FASEB J., 1996, vol. 10, p. 1485.
Mahley, R.W., Apolipoprotein E: Cholesterol Transport Protein with Expanding Role in Cell Biology, Science, 1988, vol. 240, p. 622.
Kitagawa, K., Matsumoto, M., Hori, M., and Yanagihara, T., Neuroprotective Effect of Apolipoprotein E against Ischemia, Ann. N. Y. Acad. Sci., 2002, vol. 977, p. 468.
Siest, G., Bertrand, P., Herbeth, B., et al., Apolipoprotein E Polymorphisms and Concentration in Chronic Diseases and Drug Responses, Clin. Chem. Lab. Med., 2000, vol. 38, p. 841.
Ohm, T.G., Hamker, U., Cedazo-Minguez, A., et al., Apolipoprotein E and β-A4 Amyloid: Signals and Effects, Biochem. Soc. Symp., 2001, vol. 67, p. 121.
Reiman, E.M., Caselli, R.J., Yun, L.S., et al., Preclinical Evidence of Alzheimer’s Disease in Persons Homozygous for the ε4 Allele for Apolipoprotein E, N. Engl. J. Med. 1996, vol. 334, p. 752.
Reiman, E.M., Caselli, R.J., Chen, K., et al., Declining Brain Activity in Cognitively Normal Apolipoprotein E ε4 Heterozygotes: A Foundation for Using Positron Emission Tomography Efficiently Test Treatments to Prevent Alzheimer’s Disease, Proc. Soc. Natl. Acad. Sci. USA, 2001, vol. 98, p. 3334.
Corder, E.H., Saunders, A.M., Pericak-Vance, M.A., and Roses, A.D., There Is a Pathologic Relationship between ApoE-ε4 and Alzheimer’s Disease, Arch. Neurol., 1995, vol. 52, p. 650.
Saunders, A.M., Strittmatter, W.J., Schmechel, D., et al., Association of Apolipoprotein E Allele ε4 with Late-Onset Familial and Sporadic Alzheimer’s Disease, Neurology, 1993, vol. 43, p. 1467.
Poirier, J., Delisle, M.C., Quirion, R., et al., Apolipoprotein E4 Allele As a Predictor of Cholinergic Deficits and Treatment Outcome in Alzheimer Disease, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, p. 12 260.
Farlow, M.R., Lahiri, D.K., Poirier, J., et al., Treatment Outcome of Tacrine Therapy Depends on Apolipoprotein Genotype and Gender of the Subjects with Alzheimer’s Disease, Neurology, 1998, vol. 50, p. 669.
Yaffe, K., Haan, M., and Byers, A., Estrogen Use, APOE, and Cognitive Decline: Evidence of Gene-Environment Interaction, Neurology, 2000, vol. 54, p. 1949.
Schneider, L.S. and Farlow, M., Combined Tacrine and Estrogen Replacement Therapy in Patients with Alzheimer’s Disease, Ann. N. Y. Acad. Sci., 1997, vol. 826, p. 317.
Poirier, J., Apolipoprotein E4, Cholinergic Integrity and the Pharmacogenetics of Alzheimer’s Disease, J. Psychiatry Neurosci., 1999, vol. 24, p. 147.
Pomara, N., Tun, H., Deptula, D., and Greenblatt, D.J., ApoE ε4 Allele and Susceptibility to Drug-Induced Memory Impairment in the Elderly, J. Clin. Psychopharmacol., 1998, vol. 18, p. 179.
Simon, T., Becquemont, L., Mary-Krause, M., et al., Combined Glutathione S-Transferase M1 and T1 Genetic Polymorphism and Tacrine Hepatotoxicity, Clin. Pharmacol. Ther., 2000, vol. 67, p. 432.
Schulz, J.B., Lindenau, J., Seyfried, J., and Dichgans, J., Glutathione, Oxidative Stress and Neurodegeneration, Eur. J. Biochem., 2000, vol. 267, p. 4904.
Nunomura, A., Perry, G., Aliev, G., et al., Oxidative Damage Is the Earliest Event in Alzheimer Disease, J. Neuropathol. Exp. Neurol., 2001, vol. 60, p. 759.
Piruzyan, L.A. and Piruzyan, A.L., Electron Paramagnetic Flow Dialysis (A Possible Use in Experimental Biology and Medicine), Dokl. Akad. Nauk, 2004, no. 2, p. 277.
Author information
Authors and Affiliations
Additional information
__________
Translated from Fiziologiya Cheloveka, Vol. 31, No. 4, 2005, pp. 119–130.
Original Russian Text Copyright © 2005 by Sukhanov, Ionov, Piruzyan.
Rights and permissions
About this article
Cite this article
Sukhanov, V.A., Ionov, I.D. & Piruzyan, L.A. Neurodegenerative Disorders: The Role of Genetic Factors in Their Origin and the Efficiency of Treatment. Hum Physiol 31, 472–482 (2005). https://doi.org/10.1007/s10747-005-0080-6
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10747-005-0080-6