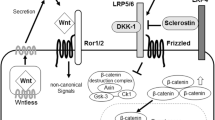
Summary
Sclerostin, the secreted protein product of the SOST gene, which is mainly expressed by osteocytes, has recently been proposed as a negative regulator of bone osteoblastogenesis. Chronic elevation of PTH reduces SOST expression by osteocytes, while controversial results have been obtained by intermittent PTH administration. We have investigated the effects of intermittently administered PTH on SOST expression and sclerostin localization, comparing them with those of controls, as they appeared in three different bone segments of rat tibia: secondary trabecular metaphyseal and epiphyseal bone, and cortical diaphyseal bone. The histomorphometric results demonstrate that PTH enhances bone turnover through anabolic effects, as shown by the association of increased bone resorption variables with a significant rise in BV/TV, Tb.Th and Tb.N and a fall in Tb.Sp. PTH induces a SOST mRNA and protein fall in secondary metaphyseal trabeculae, diaphyseal bone and in epiphyseal trabeculae. Numbers of sclerostin immunopositive osteocytes/mm2 show no change, compared with controls; there are fewer sclerostin-positive osteocytes in secondary metaphyseal trabeculae than in the other two bone areas, both in the control and PTH groups. The low numbers of sclerostin-positive osteocytes in the metaphyseal trabecular bone seem to be directly related to the fact that this area displays a high remodeling rate. The anabolic effects of PTH are in line with the fall of SOST mRNA and protein in all the three bone segments examined; the rise of bone turnover supports a negative role of SOST in bone formation.




Similar content being viewed by others
References
Bellido T, Afshan Ali A, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL (2003) Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. J Biol Chem 278:50259–50272
Bellido T , Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL (2005) Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583
Bianco P, Ballanti P, Bonucci E (1988) Tartrate-resistant acid phosphatase activity in rat osteoblasts and osteocytes. Calcif Tissue Int 43:167–71
Fermor BST (1995) PTH/PTHrP receptor expression on osteoblasts and osteocytes but not resorbing bone surfaces in growing rats. J Bone Min Res 10:1935–1943
Gardner JC, van Bezooijen RL, Mervis B, Hamdy NAT, Löwik CWGM, Hamersma H, Beighton P, Papapoulos SE (2005) Bone mineral density in sclerostosis; affected individuals and gene carriers. J Clin Endocrinol Metab 90:6392–6395
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Isogai Y, Akatsu T, Ishizuya T,Yamaguchi A, Hori M,Takahashi N, Suda T (1996) Parathyroid hormone regulates osteoblast differentiation positively or negatively depending on the differentiation stages. J Bone Min Res 11:1384–1393
Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, (2005) Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone 36:585–598
Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass II DA, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
Kolpakova E, Olsen BR (2005) Wnt/beta-catenin–a canonical tale of cell-fate choice in the vertebrate skeleton. Dev Cell 8:626–627
Langub MC, Monier-Faugere MC, Qi Q,Geng Z, Koszewski NJ, Malluche HH (2001) Parathyroid hormone/parathyroid hormone-related peptide type 1 receptor in human bone. J Bone Min Res 16:448–456
Li X, Zhang Y, Kang H, Liu W,Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887
Noda M (2006) BMP and its antagonist. Bonekey-osteovision April 3(4):5–11
Ott S (2005) Sclerostin and Wnt signaling -The pathway to bone strength. J Clin Endocrinol Metab 90:6741–6743
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325
Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S (2003) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 18:1842–1843
Robling AG, Bellido TM, Turner CH (2006) Mechanical loading reduces osteocyte expression of sclerostin protein. J Bone Miner Res Sep 21(Suppl 1):S72
Shibutani M, Uneyama C, Miyazaki K, Toyoda K, Hirose M (2000) Methacarn fixation: a novel tool for analysis of gene expressions in paraffin-embedded tissue specimens. Lab Invest 80:199–208
Silvestrini G, Ballanti P, Patacchioli FR, Mocetti P, Di Grezia R, Martin Wedard B, Angelucci L, Bonucci E (2000) Evaluation of apoptosis and the glucocorticoid receptor in the cartilage growth plate and metaphyseal bone cells of rats after high dose chronic treatment with corticosterone. Bone 26:33–42
Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever P (2005) Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J CELL SCI 119:1283–1296
Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, Latham JA (2004) Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 35:828–835
Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K (2006) Parathyroid hormone increases beta-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology 147:2583–2590
Uneyama C, Shibutani M, Masutomi N, Takagi H, Hirose M (2002) Methacarn Fixation for Genomic DNA Analysis in Microdissected, Paraffin-embedded Tissue Specimens. J Histochem Cytochem 50:1237–1245
van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H,Papapoulos SE, ten Dijke P, Lowik CW (2006a) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814
van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW (2006b) SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16:319–327
Wergedal JE, Veskovic K, Hellan M, Nyght C, Balemans W, Libanati C, Vanhoenacker FM, Tan J, Baylink DJ, Van Hul W (2003) Patients with Van Buchem Disease, an Osteosclerotic Genetic Disease, Have Elevated Bone Formation Markers, Higher Bone Density, and Greater Derived Polar Moment of Inertia than Normal. J Clin Endocrinol Metab 88:5778–5783
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Acknowledgements
This work was supported by MIUR grants to GS (COFIN) and MIUR 60% for 2004 and 2005 to FRP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silvestrini, G., Ballanti, P., Leopizzi, M. et al. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Hist 38, 261–269 (2007). https://doi.org/10.1007/s10735-007-9096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9096-3