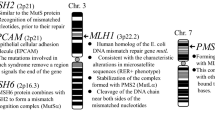
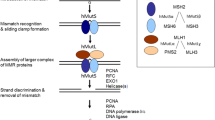
Abstract
The majority of Lynch syndrome (LS), also known as hereditary non-polyposis colorectal cancer (HNPCC), has been linked to heterozygous defects in DNA mismatch repair (MMR). MMR is a highly conserved pathway that recognizes and repairs polymerase misincorporation errors and nucleotide damage as well as functioning as a damage sensor that signals apoptosis. Loss-of-heterozygosity (LOH) that retains the mutant MMR allele and epigenetic silencing of MMR genes are associated with an increased mutation rate that drives carcinogenesis as well as microsatellite instability that is a hallmark of LS/HNPCC. Understanding the biophysical functions of the MMR components is crucial to elucidating the role of MMR in human tumorigenesis and determining the pathogenetic consequences of patients that present in the clinic with an uncharacterized variant of the MMR genes. We summarize the historical association between LS/HNPCC and MMR, discuss the mechanism of the MMR and finally examine the functional analysis of MMR defects found in LS/HNPCC patients and their relationship with the severity of the disease.


Similar content being viewed by others
References
Lynch HT (1985) Classics in oncology. Aldred Scott Warthin, M.D., Ph.D. (1866–1931). CA Cancer J Clin 35(6): 345–7
Classics in oncology. Heredity with reference to carcinoma as shown by the study of the cases examined in the pathological laboratory of the University of Michigan, 1895–1913. By Aldred Scott Warthin. 1913. CA Cancer J Clin 1985 Nov–Dec; 35(6): 348–59
Lynch HT, Smyrk T (1996) Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 78(6):1149–1167
Peltomaki P, Sistonen P, Mecklin JP et al (1992) Evidence that the MCC-APC gene region in 5q21 is not the site for susceptibility to hereditary nonpolyposis colorectal carcinoma. Cancer Res 52(16):4530–4533
Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M (1993) Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363(6429):558–561
Peltomaki P, Lothe RA, Aaltonen LA et al (1993) Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res 53(24):5853–5855
Aaltonen LA, Peltomaki P, Leach FS et al (1993) Clues to the pathogenesis of familial colorectal cancer. Science 260(5109):812–816
Levinson G, Gutman GA (1987) High frequencies of short frameshifts in poly-CA/TG tandem repeats borne by bacteriophage M13 in Escherichia coli K-12. Nucleic Acids Res 15(13):5323–5338
Reenan RA, Kolodner RD (1992) Isolation and characterization of two Saccharomyces cerevisiae genes encoding homologs of the bacterial HexA and MutS mismatch repair proteins. Genetics 132(4):963–973
Strand M, Prolla TA, Liskay RM, Petes TD (1993) Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365(6443):274–276
Fishel R, Kolodner RD (1995) Identification of mismatch repair genes and their role in the development of cancer. [Review] [158 refs]. Curr Opin Genet Dev 5(3):382–395
Kolodner R (1996) Biochemistry and genetics of eukaryotic mismatch repair. [Review] [85 refs]. Genes Dev 10(12):1433–1442
Modrich P, Lahue R (1996) Mismatch repair in replication fidelity, genetic recombination, and cancer biology. [Review] [225 refs]. Annu Rev Biochem 65:101–133
Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J (1997) Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 57(21):4749–4756
Fishel R, Lescoe MK, Rao MR et al (1993) The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75(5):1027–1038
Leach FS, Nicolaides NC, Papadopoulos N et al (1993) Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75(6):1215–1225
Bronner CE, Baker SM, Morrison PT et al (1994) Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368(6468):258–261
Papadopoulos N, Nicolaides NC, Wei YF et al (1994) Mutation of a mutL homolog in hereditary colon cancer. Science 263(5153):1625–1629
Nicolaides NC, Papadopoulos N, Liu B et al (1994) Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371(6492):75–80
Papadopoulos N, Nicolaides NC, Liu B et al (1995) Mutations of GTBP in genetically unstable cells. Science 268:1915–1917
Lipkin SM, Wang V, Jacoby R et al (2000) MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat Genet 24(1):27–35
Loeb LA (1991) Mutator phenotype may be required for multistage carcinogenesis. [Review] [63 refs]. Cancer Res 51(12):3075–3079
Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M (1994) Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet 6(3):273–281
Zhang H, Richards B, Wilson T et al (1999) Apoptosis induced by overexpression of hMSH2 or hMLH1. Cancer Res 59(13):3021–3027
Duval A, Hamelin R (2002) Genetic instability in human mismatch repair deficient cancers. Ann Genet 45(2):71–75
Duval A, Hamelin R (2002) Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res 62(9):2447–2454
Markowitz S, Wang J, Myeroff L et al (1995) Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268(5215):1336–1338
Souza RF, Appel R, Yin J et al (1996) Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet 14(3):255–257
Rampino N, Yamamoto H, Ionov Y et al (1997) Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275(5302):967–969
Boland CR, Thibodeau SN, Hamilton SR et al (1998) A national cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58(22):5248–5257
Frayling IM (1999) Microsatellite instability. Gut 45(1):1–4
Baudhuin LM, Burgart LJ, Leontovich O, Thibodeau SN (2005) Use of microsatellite instability and immunohistochemistry testing for the identification of individuals at risk for Lynch syndrome. Fam Cancer 4(3):255–265
Umar A, Boland CR, Terdiman JP et al (2004) Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Bocker T, Diermann J, Friedl W et al (1997) Microsatellite instability analysis: a multicenter study for reliability and quality control. Cancer Res 57(21):4739–4743
Roger M (1972) Evidence for conversion of heteroduplex transforming DNAs to homoduplex by recipient pneumococcal cells. Proc Nat Acad Sci USA 69:466–470
Tiraby J-G, Fox MS (1973) Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci USA 70:3541–3545
Wildenberg J, Meselson M (1975) Mismatch repair in heteroduplex DNA. Proc Natl Acad Sci USA 72(6):2202–2206
Marinus MG (1976) Adenine methylation of Okazaki fragments in Escherichia coli. J Bacteriol 128(3):853–854
Radman M, Wagner RE, Glickman BW, Meselson M (1980) DNA methylation, mismatch correction and genetic stability. In: Alacevic M (ed) Progress in environmental mutagenesis. Elsevier/North Holland Biomedical Press, Amsterdam, pp 121–130
Siegel EC, Bryson V (1967) Mutator gene of Escherichia coli B. J Bacteriol 94:38–47
Goldstein A, Smoot JS (1955) A strain of Escherichia coli with an unusually high rate of auxotrophic mutation. J Bacteriol 70:588–595
Hill RF (1970) Location of genes controlling excision repair of UV damage and mutator activity in Escherichia coli WP2. Mutat Res 9(3):341–344
Marinus MG (1973) Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet 127(1):47–55
Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T (2006) DNA repair and mutagenesis, 2nd edn. American Society of Microbiology, Washington
Iyer RR, Pluciennik A, Burdett V, Modrich PL (2006) DNA mismatch repair: functions, mechanisms. Chem Rev 106(2):302–323
Jascur T, Boland CR (2006) Structure and function of the components of the human DNA mismatch repair system. Int J Cancer 119(9):2030–2035
Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7(5):335–346
Welsh KM, Lu AL, Clark S, Modrich P (1987) Isolation and characterization of the Escherichia coli mutH gene product. J Biol Chem 262(32):15624–15629
Constantin N, Dzantiev L, Kadyrov FA, Modrich P (2005) Human mismatch repair: reconstitution of a nick-directed bidirectional reaction. J Biol Chem 280(48):39752–39761
Grilley M, Griffith J, Modrich P (1993) Bidirectional excision in methyl-directed mismatch repair. J Biol Chem 268(16):11830–11837
Kolodner RD, Mendillo ML, Putnam CD (2007) Coupling distant sites in DNA during DNA mismatch repair. Proc Natl Acad Sci USA 104(32):12953–12954
Junop MS, Obmolova G, Rausch K, Hsieh P, Yang W (2001) Composite active site of an ABC ATPase: MutS uses ATP to verify mismatch recognition and authorize DNA repair. Mol Cell 7(1):1–12
Allen DJ, Makhov A, Grilley M et al (1997) MutS mediates heteroduplex loop formation by a translocation mechanism. EMBO J 16(14):4467–4476
Blackwell LJ, Bjornson KP, Modrich P (1998) DNA-dependent activation of the hMutS alpha ATPase. J Biol Chem 273(48):32049–32054
Acharya S, Foster PL, Brooks P, Fishel R (2003) The coordinated functions of the E coli MutS and MutL proteins in mismatch repair. Mol Cell 12(1):233–246
Fishel R (1999) Signaling mismatch repair in cancer. Nat Med 5(11):1239–1241
Gradia S, Acharya S, Fishel R (1997) The human mismatch recognition complex hMSH2–hMSH6 functions as a novel molecular switch. Cell 91(7):995–1005
Gradia S, Subramanian D, Wilson T et al (1999) hMSH2–hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol Cell 3(2):255–261
Pluciennik A, Modrich P (2007) Protein roadblocks and helix discontinuities are barriers to the initiation of mismatch repair. Proc Natl Acad Sci USA 104(31):12709–12713
Cho WK, Jeong C, Kim D et al (2012) ATP alters the diffusion mechanics of MutS on mismatched DNA. Structure 20(7):1264–1274
Jeong C, Cho WK, Song KM et al (2011) MutS switches between two fundamentally distinct clamps during mismatch repair. Nat Struct Mol Biol 18(3):379–385
Fishel R, Acharya S, Berardini M et al (2000) Signaling mismatch repair: the mechanics of an adenosine-nucleotide molecular switch. Cold Spring Harb Symp Quant Biol 65:217–224
Guerrette S, Wilson T, Gradia S, Fishel R (1998) Interactions of human hMSH2 with hMSH3 and hMSH2 with hMSH6: examination of mutations found in hereditary nonpolyposis colorectal cancer. Mol Cell Biol 18(11):6616–6623
Fishel R, Wilson T (1997) MutS homologs in mammalian cells. [Review] [84 refs]. Curr Opin Genet Dev 7(1):105–113
Antony E, Hingorani MM (2004) Asymmetric ATP binding and hydrolysis activity of the Thermus aquaticus MutS dimer is key to modulation of its interactions with mismatched DNA. Biochemistry 43:13115–13128
Antony E, Khubchandani S, Chen S, Hingorani MM (2006) Contribution of Msh2 and Msh6 subunits to the asymmetric ATPase and DNA mismatch binding activities of Saccharomyces cerevisiae Msh2–Msh6 mismatch repair protein. DNA Repair (Amst) 5(2):153–162
Heinen CD, Cyr JL, Cook C et al (2011) Human MSH2 (hMSH2) protein controls ATP processing by hMSH2–hMSH6. J Biol Chem 286(46):40287–40295
Mazur DJ, Mendillo ML, Kolodner RD (2006) Inhibition of Msh6 ATPase activity by mispaired DNA induces a Msh2(ATP)-Msh6(ATP) state capable of hydrolysis-independent movement along DNA. Mol Cell 22(1):39–49
Fishel R (1998) Mismatch repair, molecular switches, and signal transduction. [Review] [56 refs]. Genes Dev 12(14):2096–2101
Gradia S, Acharya S, Fishel R (2000) The role of mismatched nucleotides in activating the hMSH2–hMSH6 molecular switch. J Biol Chem 275:3922–3930
Snowden T, Acharya S, Butz C, Berardini M, Fishel R (2004) hMSH4–hMSH5 recognizes holliday junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 15(3):437–451
Gorman J, Chowdhury A, Surtees JA et al (2007) Dynamic basis for one-dimensional DNA scanning by the mismatch repair complex Msh2–Msh6. Mol Cell 28(3):359–370
Gorman J, Plys AJ, Visnapuu ML, Alani E, Greene EC (2010) Visualizing one-dimensional diffusion of eukaryotic DNA repair factors along a chromatin lattice. Nat Struct Mol Biol 17(8):932–938
Li F, Tian L, Gu L, Li GM (2009) Evidence that nucleosomes inhibit mismatch repair in eukaryotic cells. J Biol Chem 284(48):33056–33061
Kunkel TA, Erie DA (2005) DNA mismatch repair. Annu Rev Biochem 74:681–710
Sass LE, Lanyi C, Weninger K, Erie DA (2011) Single-molecule FRET TACKLE reveals highly dynamic mismatched DNA-MutS complexes. Biochemistry 49(14):3174–3190
Mazurek A, Johnson CN, Germann MW, Fishel R (2009) Sequence context effect for hMSH2–hMSH6 mismatch-dependent activation. Proc Natl Acad Sci USA 106(11):4177–4182
Obmolova G, Ban C, Hsieh P, Yang W (2000) Crystal structures of mismatch repair protein MutS, its complex with a substrate DNA [see comments]. Nature 407(6805):703–710
Lamers MH, Perrakis A, Enzlin JH, Winterwerp HH, de Wind N, Sixma TK (2000) The crystal structure of DNA mismatch repair protein MutS binding to a G x T mismatch. [see comments]. Nature 407(6805):711–717
Warren JJ, Pohlhaus TJ, Changela A, Iyer RR, Modrich PL, Beese LS (2007) Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell 26(4):579–592
Javaid S, Manohar M, Punja N et al (2009) Nucleosome remodeling by hMSH2–hMSH6. Mol Cell 36(6):1086–1094
Guerrette S, Acharya S, Fishel R (1999) The interaction of the human MutL homologues in hereditary nonpolyposis colon cancer. J Biol Chem 274(10):6336–6341
Dutta R, Inouye M (2000) GHKL, An emergent ATPase/kinase superfamily. Trends Biochem Sci 25(1):24–28
Ban C, Yang W (1998) Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell 95(4):541–552
Sacho EJ, Kadyrov FA, Modrich P, Kunkel TA, Erie DA (2008) Direct visualization of asymmetric adenine nucleotide-induced conformational changes in Mutlalpha. Mol Cell 29(1):112–121
Bende SM, Grafstrom RH (1991) The DNA binding properties of the MutL protein isolated from Escherichia coli. Nucleic Acids Res 19:1549–1555
Drotschmann K, Hall MC, Shcherbakova PV et al (2002) DNA binding properties of the yeast Msh2–Msh6, Mlh1-Pms1 heterodimers. Biol Chem 383(6):969–975
Park J, Jeon Y, In D, Fishel R, Ban C, Lee JB (2010) Single-molecule analysis reveals the kinetics and physiological relevance of MutL-ssDNA binding. PLoS ONE 5(11):e15496
Kadyrov FA, Dzantiev L, Constantin N, Modrich P (2006) Endonucleolytic function of MutLalpha in human mismatch repair. Cell 126(2):297–308
Kadyrov FA, Holmes SF, Arana ME et al (2007) Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease. J Biol Chem 282(51):37181–37190
Kosinski J, Plotz G, Guarne A, Bujnicki JM, Friedhoff P (2008) The PMS2 subunit of human MutLalpha contains a metal ion binding domain of the iron-dependent repressor protein family. J Mol Biol 382(3):610–627
Pillon MC, Lorenowicz JJ, Uckelmann M et al (2010) Structure of the endonuclease domain of MutL: unlicensed to cut. Mol Cell 39(1):145–151
Pillon MC, Miller JH, Guarne A (2010) The endonuclease domain of MutL interacts with the beta sliding clamp. DNA Repair (Amst) 10(1):87–93
Grilley M, Welsh KM, Su SS, Modrich P (1989) Isolation and characterization of the Escherichia coli mutL gene product. J Biol Chem 264(2):1000–1004
Mendillo ML, Mazur DJ, Kolodner RD (2005) Analysis of the interaction between the Saccharomyces cerevisiae MSH2–MSH6 and MLH1–PMS1 complexes with DNA using a reversible DNA end-blocking system. J Biol Chem 280(23):22245–22257
Schofield MJ, Nayak S, Scott TH, Du C, Hsieh P (2001) Interaction of Escherichia coli MutS and MutL at a DNA mismatch. J Biol Chem 276(30):28291–28299
Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD (2011) Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell 147(5):1040–1053
Lopez de Saro FJ, Marinus MG, Modrich P, O’Donnell M (2006) The beta sliding clamp binds to multiple sites within MutL and MutS. J Biol Chem 281(20):14340–14349
Viswanathan M, Lovett ST (1998) Single-strand DNA-specific exonucleases in Escherichia coli—roles in repair and mutation avoidance. Genetics 149(1):7–16
Pluciennik A, Burdett V, Lukianova O, O’Donnell M, Modrich P (2009) Involvement of the beta clamp in methyl-directed mismatch repair in vitro. J Biol Chem 284(47):32782–32791
Ramilo C, Gu L, Guo S et al (2002) Partial reconstitution of human DNA mismatch repair in vitro: characterization of the role of human replication protein A. Mol Cell Biol 22(7):2037–2046
Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, Modrich P (2010) PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc Natl Acad Sci USA 107(37):16066–16071
Zhang Y, Yuan F, Presnell SR et al (2005) Reconstitution of 5′-directed human mismatch repair in a purified system. Cell 122(5):693–705
Belvederesi L, Bianchi F, Galizia E et al (2008) MSH2 missense mutations, HNPCC syndrome: pathogenicity assessment in a human expression system. Hum Mutat 29(11):E296–E309
Hardt K, Heick SB, Betz B et al (2011) Missense variants in hMLH1 identified in patients from the German HNPCC consortium, functional studies. Fam Cancer 10(2):273–284
Kondo E, Suzuki H, Horii A, Fukushige S (2003) A yeast two-hybrid assay provides a simple way to evaluate the vast majority of hMLH1 germ-line mutations. Cancer Res 63(12):3302–3308
Schmutte C, Marinescu RC, Sadoff MM, Guerrette S, Overhauser J, Fishel R (1998) Human exonuclease I interacts with the mismatch repair protein hMSH2. Cancer Res 58(20):4537–4542
Schmutte C, Sadoff MM, Shim KS, Acharya S, Fishel R (2001) The interaction of DNA mismatch repair proteins with human exonuclease I. J Biol Chem 276(35):33011–33018
Raevaara TE, Korhonen MK, Lohi H et al (2005) Functional significance, clinical phenotype of nontruncating mismatch repair variants of MLH1. Gastroenterology 129(2):537–549
Takahashi M, Shimodaira H, Andreutti-Zaugg C, Iggo R, Kolodner RD, Ishioka C (2007) Functional analysis of human MLH1 variants using yeast and in vitro mismatch repair assays. Cancer Res 67(10):4595–4604
Heinen CD, Wilson T, Mazurek A, Berardini M, Butz C, Fishel R (2002) HNPCC mutations in hMSH2 result in reduced hMSH2–hMSH6 molecular switch functions. Cancer Cell 1:469–478
Brieger A, Plotz G, Raedle J et al (2005) Characterization of the nuclear import of human MutLalpha. Mol Carcinog 43(1):51–58
Lei X, Zhu Y, Tomkinson A, Sun L (2004) Measurement of DNA mismatch repair activity in live cells. Nucleic Acids Res 32(12):e100
Ollila S, Dermadi Bebek D, Jiricny J, Nystrom M (2008) Mechanisms of pathogenicity in human MSH2 missense mutants. Hum Mutat 29(11):1355–1363
Trojan J, Zeuzem S, Randolph A et al (2002) Functional analysis of hMLH1 variants, HNPCC-related mutations using a human expression system. Gastroenterology 122(1):211–219
Naruse H, Ikawa N, Yamaguchi K et al (2009) Determination of splice-site mutations in Lynch syndrome (hereditary non-polyposis colorectal cancer) patients using functional splicing assay. Fam Cancer 8(4):509–517
Tournier I, Vezain M, Martins A et al (2008) A large fraction of unclassified variants of the mismatch repair genes MLH1, MSH2 is associated with splicing defects. Hum Mutat 29(12):1412–1424
Fishel R (2001) The selection for mismatch repair defects in hereditary nonpolyposis colorectal cancer: revising the mutator hypothesis. Cancer Res 61(20):7369–7374
Gong JG, Costanzo A, Yang HQ et al (1999) The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage [see comments]. Nature 399(6738):806–809
Jain A, Liu R, Ramani B et al (2011) Probing cellular protein complexes using single-molecule pull-down. Nature 473(7348):484–488
Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T (2008) Advances in single-molecule fluorescence methods for molecular biology. Annu Rev Biochem 77:51–76
Roy R, Hohng S, Ha T (2008) A practical guide to single-molecule FRET. Nat Methods 5(6):507–516
Yoshioka K, Yoshioka Y, Hsieh P (2006) ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol Cell 22(4):501–510
Acknowledgments
The authors would like to thank Christopher Heinen and members of the Fishel Laboratory for helpful discussions. This work was supported by NIH Grant CA67007.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martín-López, J.V., Fishel, R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Familial Cancer 12, 159–168 (2013). https://doi.org/10.1007/s10689-013-9635-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-013-9635-x