Abstract
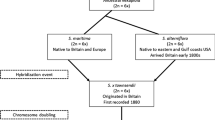
Interactions between insect herbivores and host plants can involve herbivore-host pairs that are evolutionarily ancient or only recently associated. Novel herbivore-host species pairs are continually being formed via host shifts, dispersal, and increasingly via anthropogenic introductions. Conceptual models of enemy-victim coevolution (specifically, the evolution of plant tolerance and of insect virulence) suggest that the impact of an herbivore on its novel host should, at least at first, be more intense than its impact on its ancestral host. We tested this hypothesis for the specialist gallmaking caterpillar Gnorimoschema gallaesolidaginis (Lepidoptera: Gelechiidae) on its ancestral and novel hosts, Solidago altissima and S. gigantea. We measured aboveground ramet mass for paired attacked and unattacked ramets of each species at two sites (Fredericton, NB, and Toronto, ON, Canada), and also measured allocation of tissue mass to stems, leaves, and flowers in galled and ungalled ramets. G. gallaesolidaginis attack reduced ramet growth considerably more on S. gigantea, the novel host, consistent with the coevolutionary hypothesis. We were unable to detect reallocation of tissues in galled ramets as a mechanism for tolerance, and found no intraspecific difference in the impact of gallmaking on allocation patterns. Herbivore host shifts between alternative native hosts will provide an excellent opportunity to understand the evolutionary history of novel herbivore-host associations, particularly (as in the Solidago system) when multiple insect herbivores have host-shifted across the same plant pair.


Similar content being viewed by others
Notes
In the insect-plant interactions literature, the word "virulence" is used in two very different senses. It can mean the ability of an insect to overcome plant defences (to overcome resistance), or it can mean the damage inflicted on the plant during the insect's attack (Wilhoit and Gould 1992). We are concerned here with the latter sense.
References
Abhilasha D, Joshi J (2009) Enhanced fitness due to higher fecundity, increased defence against a specialist and tolerance towards a generalist herbivore in an invasive annual plant. J Plant Ecol 2:77–86
Abrahamson WG, Weis AE (1997) Evolutionary ecology across three trophic levels: goldenrods, gallmakers, and natural enemies. Princeton University Press, Princeton
Agrawal AA (2011) Current trends in the evolutionary ecology of plant defence. Funct Ecol 25:420–432
Alizon S, Hurford A, Mideo N, van Baalen M (2009) Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 22:245–259
Bertheau C, Brockerhoff EG, Roux-Morabito G, Lieutier F, Jactel H (2010) Novel insect-tree associations resulting from accidental and intentional biological ‘invasions’: a meta-analysis of effects on insect fitness. Ecol Lett 13:506–515
Carroll SP, Dingle H, Klassen SP (1997) Genetic differentiation of fitness-associated traits among rapidly evolving populations of the soapberry bug. Evolution 51:1182–1188
Carroll SP, Loye JE, Dingle H, Mathieson M, Famula TR, Zalucki MP (2005) And the beak shall inherit—evolution in response to invasion. Ecol Lett 8:944–951
Chun YJ, van Kleunen M, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13:937–946
Diegisser T, Tritsch C, Seitz A, Johannesen J (2009) Infestation of a novel host plant by Tephritis conura (Diptera: Tephritidae) in northern Britain: host-range expansion or host shift? Genetica 137:87–97
Dres M, Mallet J (2002) Host races in plant-feeding insects and their importance in sympatric speciation. Phil Trans R Soc Lond B 357:471–492
Ewald PW (1994) Evolution of infectious disease. Oxford University Press, New York
Feder JL, Berlocher SH, Opp SB (1998) Sympatric host-race formation and speciation in Rhagoletis (Diptera: Tephritidae): a tale of two species for Charles D. In: Mopper S (ed) Genetic structure and local adaptation in natural insect populations. Chapman and Hall, New York, pp 408–441
Ferrari J, Via S, Godfray HCJ (2008) Population differentiation and genetic variation in performance on eight hosts in the pea aphid complex. Evolution 62:2508–2524
Fontes EMG, Habeck DH, Slansky F Jr (1994) Phytophagous insects associated with goldenrods (Solidago spp.) in Gainesville, Florida. Florida Entomol 77:209–221
Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Funct Ecol 25:399–407
Fornoni J, Núñez-Farfán J, Valverde PL, Rausher MD (2004) Evolution of mixed strategies of plant defense allocation against natural enemies. Evolution 58:1685–1695
Graves SD, Shapiro AM (2003) Exotics as host plants of the California butterfly fauna. Biol Conserv 110:413–433
Hakes AS, Cronin JT (2011) Resistance and tolerance to herbivory in Solidago altissima (Asteraceae): genetic variability, costs, and selection for multiple traits. Am J Bot 98:1446–1455
Hartnett DC, Abrahamson WG (1979) The effects of stem gall insects on life history patterns in Solidago canadensis. Ecology 60:910–917
Heard SB, Ancheta J (2011) Impacts of insect herbivores on rare plant populations. Biol Conserv 144:2395–2402
Heard SB, Cox GH (2009) Plant module size and attack by the goldenrod spindle-gall moth. Can Entomol 141:406–414
Heard SB, Stireman JO III, Nason JD, Cox GH, Kolacz CR, Brown JM (2006) On the elusiveness of enemy-free space: spatial, temporal, and host-plant-related variation in parasitoid attack rates on three gallmakers of goldenrods. Oecologia 150:421–434
Hokkanen HMT, Pimentel D (1989) New Associations in biological control—theory and practice. Can Entomol 121:829–840
Huang W, Siemann E, Wheeler GS, Zou JW, Carrillo J, Ding JQ (2010) Resource allocation to defence and growth are driven by different responses to generalist and specialist herbivory in an invasive plant. J Ecol 98:1157–1167
Jokela J, Schmid-Hempel P, Rigby MC (2000) Dr. Pangloss restrained by the Red Queen—steps towards a unified defence theory. Oikos 89:267–274
Kato T, Bonet A, Yoshitake H, Romero-Napoles J, Jinbo U, Ito M, Shimada M (2010) Evolution of host utilization patterns in the seed beetle genus Mimosestes Bridwell (Coleoptera: Chrysomelidae: Bruchinae). Mol Phylog Evol 55:816–832
Lau JA (2008) Beyond the ecological: Biological invasions alter natural selection on a native plant species. Ecology 89:1023–1031
Lehnertz EA (1999) Subsampling methods for measuring the fitness of Solidago altissima. B.Sc. (Hons) Thesis, University of Iowa
Leiby RW (1922) The biology of the goldenrod gall-maker Gnorimoschema gallaesolidaginis. J NY Entomol Soc 30:81–95
Levin BR, Bull JJ (1994) Short-sighted evolution and the virulence of pathogenic microorganisms. Trends Microbiol 2:76–81
Levin S, Pimentel D (1981) Selection of intermediate rates of increase in parasite-host systems. Amer Nat 117:308–315
Levin BR, Allison AC, Bremermann HJ, Cavalli-Sforza LL, Clarke BC, Frentzel-Beyme R, Hamilton WD, Levin SA, May RM, Thieme HR (1982) Evolution of parasites and hosts. In: Anderson RM, May RM (eds) Population biology of infectious diseases. Springer, Berlin, pp 214–243
Logarzo GA, Casalinuovo MA, Piccinali RV, Braun K, Hasson E (2011) Geographic host use variability and host range evolutionary dynamics in the phytophagous insect Apagomerella versicolor (Cerambycidae). Oecologia 165:387–402
Maddox GD, Root RB (1990) Structure of the encounter between goldenrod (Solidago altissima) and its diverse insect fauna. Ecology 71:2115–2124
Maddox GD, Cook RE, Wimberger PH, Gardescu S (1989) Clone structure in four Solidago altissima (Asteraceae) populations: rhizome connections within genotypes. Am J Bot 76:318–326
Maron JL, Vila M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Matsubayashi KW, Ohshima I, Nosil P (2010) Ecological speciation in phytophagous insects. Entomol Exper Appl 134:1–27
Messenger SL, Molineux IJ, Bull JJ (1999) Virulence evolution in a virus obeys a trade-off. Proc Roy Soc Lond B 266:397–404
Miller WE (2000) A comparative taxonomic-natural history study of eight Nearctic species of Gnorimoschema that induce stem galls on Asteraceae, including descriptions of three new species (Lepidoptera: Gelechiidae). Entomological Society of America, Lanham
Mosquera J, Adler FR (1998) Evolution of virulence: a unified framework for coinfection and superinfection. J Theor Biol 195:293–313
Nason JD, Heard SB, Williams FR (2002) Host associated genetic differentiation in the goldenrod elliptical-gall moth, Gnorimoschema gallaesolidaginis (Lepidoptera: Gelechiidae). Evolution 56:1475–1488
Nosil P (2007) Divergent host plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking sticks. Amer Nat 169:151–162
Nowak MA, May RM (1994) Superinfection and the evolution of parasite virulence. Proc R Soc Lond B 255:81–89
Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38:541–566
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461
Peńa C, Wahlberg N (2008) Prehistorical climate change increased diversification of a group of butterflies. Biol Lett 4:274–278
Percy DM, Page RDM, Cronk QCB (2004) Plant-insect interactions: double-dating associated insect and plant lineages reveals asynchronous radiations. Syst Biol 53:120–127
Pimentel D (1963) Introducing parasites and predators to control native pests. Can Entomol 95:785–792
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rebek EJ, Herms DA, Smitley DR (2008) Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp.). Envir Entomol 37:242–246
Restif O, Koella JC (2004) Concurrent evolution of resistance and tolerance to pathogens. Amer Nat 164:E90–E102
Rohr JR, Raffel TR, Hall CA (2010) Developmental variation in resistance and tolerance in a multi-host-parasite system. Funct Ecol 24:1110–1121
Root RB, Cappuccino N (1992) Patterns in population change and the organization of the insect community associated with goldenrod. Ecol Monogr 63:393–420
Roy BA, Kirchner JW (2000) Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54:51–63
Schmid B, Puttick GM, Burgess KH, Bazzaz FA (1988a) Clonal integration and effects of simulated herbivory in old-field perennials. Oecologia 75:465–471
Schmid B, Puttick GM, Burgess KH, Bazzaz FA (1988b) Correlations between genet architecture and some life history features in three species of Solidago. Oecologia 75:459–464
Seehawer JM (2002) Impact of larval phenology and fitness trade-offs on the host races of Gnorimoschema gallaesolidaginis and host related population structure in one of its parasitoids, Copidosoma gelechiae. M.Sc. Thesis, University of Iowa
Semple JC, Cook RE (2006) Solidago. In: Flora North America Editorial Committee (ed) Flora of North America. Oxford University Press, Oxford, pp 107–166
Singer MC, Wee B, Hawkins S, Butcher M (2008) Rapid natural and anthropogenic diet evolution: three examples from checkerspot butterflies. In: Tilmon KJ (ed) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects University of California Press. Berkeley, CA, pp 311–324
Stastny M, Battisti A, Petrucco-Toffolo E, Schlyter F, Larsson S (2006) Host-plant use in the range expansion of the pine processionary moth, Thaumetopoea pityocampa. Ecol Entomol 31:481–490
Stireman JO III, Nason JD, Heard SB (2005) Host-associated genetic differentiation in phytophagous insects: general phenomenon or isolated exceptions? Evidence from a goldenrod insect community. Evolution 59:2573–2587
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Vigneux F, Bashey F, Sicard M, Lively C (2008) Low migration decreases interference competition among parasites and increases virulence. J Evol Biol 21:1245–1251
Wilhoit LR, Gould F (1992) Evolution of herbivore virulence to plant resistance: influence of variety mixtures. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens: ecology, evolution and genetics. University of Chicago Press, Chicago, pp 91–119
Winkler IS, Mitter C, Scheffer SJ (2009) Repeated climate-linked host shifts have promoted diversification in a temperate clade of leaf-mining flies. Proc Natl Acad Sci USA 106:18103–18108
Acknowledgments
We thank Peter Sykes for work on lab techniques, Mickey Eubanks for sharing relevant field protocols, Graham Cox for helping with field collections, and Anurag Agrawal, John Endler, Andrew Hendry, and two anonymous reviewers for constructive comments on the manuscript. We are grateful to Tommy Thompson Park and the City of Fredericton for permission to collect. This work was supported by grants to SBH from the Natural Sciences and Engineering Research Council (Canada; Discovery Grants) and the National Science Foundation (USA; DEB 0107752).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heard, S.B., Kitts, E.K. Impact of attack by Gnorimoschema gallmakers on their ancestral and novel Solidago hosts. Evol Ecol 26, 879–892 (2012). https://doi.org/10.1007/s10682-011-9545-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9545-z