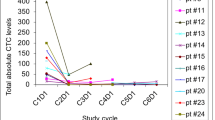
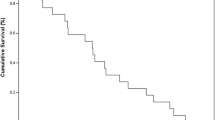
Summary
Background Activation of the vascular endothelial growth factor receptor (VEGFR) and the oncogenic Src pathway has been implicated in the development of castration-resistant prostate cancer (CRPC) in preclinical models. Cediranib and dasatinib are multi-kinase inhibitors targeting VEGFR and Src respectively. Phase II studies of cediranib and dasatinib in CRPC have shown single agent activity. Methods Docetaxel-pretreated CRPC patients were randomized to arm A: cediranib alone (20 mg/day) versus arm B: cediranib (20 mg/day) plus dasatinib (100 mg/day) given orally on 4-week cycles. Primary endpoint was 12-week progression-free survival (PFS) as per the Prostate Cancer Clinical Trials Working Group (PCWG2). Patient reported outcomes were evaluated using Functional Assessment of Cancer Therapy-Prostate (FACT-P) and Present Pain Intensity (PPI) scales. Correlative studies of bone turnover markers (BTM), including bone alkaline phosphate (BAP) and serum beta-C telopeptide (B-CTx) were serially assayed. Results A total of 22 patients, 11 per arm, were enrolled. Baseline demographics were similar in both arms. Median number of cycles =4 in arm A (range 1–12) and 2 in arm B (range 1–9). Twelve-week PFS was 73 % in arm A versus 18 % in arm B (p = 0.03). Median PFS in months (arm A versus B) was: 5.2 versus 2.6 (95 % CI: 1.9–6.5 versus 1.4-not reached). Most common grade 3 toxicities were hypertension, anemia and thrombocytopenia in arm A and hypertension, diarrhea and fatigue in arm B. One treatment-related death (retroperitoneal hemorrhage) was seen in arm A. FACT-P and PPI scores did not significantly change in either arm. No correlation between BTM and PFS was seen in either arm. Conclusions Although limited by small numbers, this randomized study showed that the combination of VEGFR and Src targeted therapy did not result in improved efficacy and may be associated with a worse outcome than VEGFR targeted therapy alone in patients with CRPC. ClinicalTrials.gov number: NCT01260688.




Similar content being viewed by others
References
Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol Off J Am Soc Clin Oncol 26(2):242–245
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351(15):1513–1520
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15):1502–1512
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376(9747):1147–1154
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S et al (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368(2):138–148
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F et al (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13(10):983–992
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367(13):1187–1197
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M et al (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369(3):213–223
Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA (2009) Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res 69(11):4708–4715
Talagas M, Uguen A, Garlantezec R, Fournier G, Doucet L, Gobin E, Marcorelles P, Volant A, DE Braekeleer M (2013) VEGFR1 and NRP1 endothelial expressions predict distant relapse after radical prostatectomy in clinically localized prostate cancer. Anticancer Res 33(5):2065–2075
George DJ, Halabi S, Shepard TF, Vogelzang NJ, Hayes DF, Small EJ, Kantoff PW (2001) Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on cancer and leukemia Group B 9480. Clin Cancer Res Off J Am Assoc Cancer Res 7(7):1932–1936
Cai H, Babic I, Wei X, Huang J, Witte ON (2011) Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res 71(3):862–872
Zhoul J, Hernandez G, Tu SW, Huang CL, Tseng CP, Hsieh JT (2005) The role of DOC-2/DAB2 in modulating androgen receptor-mediated cell growth via the nongenomic c-Src-mediated pathway in normal prostatic epithelium and cancer. Cancer Res 65(21):9906–9913
Martin GS (2001) The hunting of the Src. Nat Rev Mol Cell Biol 2(6):467–475
Yeatman TJ (2004) A renaissance for SRC. Nat Rev Cancer 4(6):470–480
Stewart RJ, Panigrahy D, Flynn E, Folkman J (2001) Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol 165(2):688–693
Mabjeesh NJ, Willard MT, Frederickson CE, Zhong H, Simons JW (2003) Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B in prostate cancer cells. Clin Cancer Res Off J Am Assoc Cancer Res 9(7):2416–2425
Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R (2008) The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell 19(1):394–404
Yang JC, Bai L, Yap S, Gao AC, Kung HJ, Evans CP (2010) Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC-3 bone model. Mol Cancer Ther 9(6):1629–1637
Mohamedali KA, Li ZG, Starbuck MW, Wan X, Yang J, Kim S, Zhang W, Rosenblum MG, Navone NM (2011) Inhibition of prostate cancer osteoblastic progression with VEGF121/rGel, a single agent targeting osteoblasts, osteoclasts, and tumor neovasculature. Clin Cancer Res Off J Am Assoc Cancer Res 17(8):2328–2338
Kitagawa Y, Dai J, Zhang J, Keller JM, Nor J, Yao Z, Keller ET (2005) Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res 65(23):10921–10929
Vessella RL, Corey E (2006) Targeting factors involved in bone remodeling as treatment strategies in prostate cancer bone metastasis. Clin Cancer Res Off J Am Assoc Cancer Res 12(20 Pt 2):6285s–6290s
Blanchard F, Duplomb L, Baud’huin M, Brounais B (2009) The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev 20(1):19–28
Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP (1995) Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375(6532):577–581
Kendrew J, Odedra R, Logie A, Taylor PJ, Pearsall S, Ogilvie DJ, Wedge SR, Jurgensmeier JM (2013) Anti-tumour and anti-vascular effects of cediranib (AZD2171) alone and in combination with other anti-tumour therapies. Cancer Chemother Pharmacol 71(4):1021–1032
Trarbach T, Schultheis B, Gauler TC, Schneider V, Strumberg D, Eberhardt WE, Le Scouiller S, Marotti M, Brown KH, Drevs J (2012) Phase I open-label study of cediranib, an oral inhibitor of VEGF signalling, in combination with the oral Src inhibitor saracatinib in patients with advanced solid tumours. Investig New Drugs 30(5):1962–1971
Trarbach TDJ, Strumberg D, Gauler TC, Schneider V, Eberhardt WE, Marotti M, Puchalski TA, Swaisland AJ (2008) A phase I, open-label, multicenter study of cediranib and AZD0530 in patients with advanced solid tumors. In: ASCO Annual Meeting: 2008; Chicago
Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, Yamada Y, Tamura T, Fukuoka K, Kimura H et al (2007) AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res Off J Am Assoc Cancer Res 13(10):3051–3057
Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO et al (2005) AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65(10):4389–4400
Morelli MP, Brown AM, Pitts TM, Tentler JJ, Ciardiello F, Ryan A, Jurgensmeier JM, Eckhardt SG (2009) Targeting vascular endothelial growth factor receptor-1 and −3 with cediranib (AZD2171): effects on migration and invasion of gastrointestinal cancer cell lines. Mol Cancer Ther 8(9):2546–2558
Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J, Kim HR (2010) A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res 70(23):9631–9640
Ustach CV, Taube ME, Hurst NJ Jr, Bhagat S, Bonfil RD, Cher ML, Schuger L, Kim HR (2004) A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res 64(5):1722–1729
Najy AJ, Jung YS, Won JJ, Conley-LaComb MK, Saliganan A, Kim CJ, Heath E, Cher ML, Bonfil RD, Kim HR (2012) Cediranib inhibits both the intraosseous growth of PDGF D-positive prostate cancer cells and the associated bone reaction. Prostate 72(12):1328–1338
Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM et al (2007) Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol Off J Am Soc Clin Oncol 25(21):3045–3054
Sahebjam S, Bedard PL, Castonguay V, Chen Z, Reedijk M, Liu G, Cohen B, Zhang WJ, Clarke B, Zhang T et al (2013) A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503). Br J Cancer 109(4):943–949
Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D et al (2008) Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol Off J Am Soc Clin Oncol 26(11):1871–1878
Fiedler W, Mesters R, Heuser M, Ehninger G, Berdel WE, Zirrgiebel U, Robertson JD, Puchalski TA, Collins B, Jurgensmeier JM et al (2010) An open-label, Phase I study of cediranib (RECENTIN) in patients with acute myeloid leukemia. Leuk Res 34(2):196–202
Ryan CJ, Stadler WM, Roth B, Hutcheon D, Conry S, Puchalski T, Morris C, Small EJ (2007) Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC). Investig New Drugs 25(5):445–451
Dahut WL, Madan RA, Karakunnel JJ, Adelberg D, Gulley JL, Turkbey IB, Chau CH, Spencer SD, Mulquin M, Wright J et al (2013) Phase II clinical trial of cediranib in patients with metastatic castration-resistant prostate cancer. BJU Int 111(8):1269–1280
Sridhar SS, Mackenzie MJ, Hotte SJ, Mukherjee SD, Tannock IF, Murray N, Kollmannsberger C, Haider MA, Chen EX, Halford R et al (2013) A phase II study of cediranib (AZD 2171) in treatment naive patients with progressive unresectable recurrent or metastatic renal cell carcinoma. A trial of the PMH phase 2 consortium. Investig New Drugs 31(4):1008–1015
Mulders P, Hawkins R, Nathan P, de Jong I, Osanto S, Porfiri E, Protheroe A, van Herpen CM, Mookerjee B, Pike L et al (2012) Cediranib monotherapy in patients with advanced renal cell carcinoma: results of a randomised phase II study. Eur J Cancer 48(4):527–537
Ledermann JA PT, Raja FA, Embleton A, Rustin GJS, Jayson G, Kaye SB, Swart AM, Vaughan M, Hirte H (2013) Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: Results of the ICON6 trial. In: ECCO, The European Cancer Congress 2013: 2013; Amsterdam
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM et al (2004) Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47(27):6658–6661
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R et al (2010) Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 362(24):2260–2270
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipina JJ, Kim DW, Ogura M et al (2012) Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 119(5):1123–1129
Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE (2008) Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res 68(9):3323–3333
Rice L, Lepler S, Pampo C, Siemann DW (2012) Impact of the SRC inhibitor dasatinib on the metastatic phenotype of human prostate cancer cells. Clin Exp Metastasis 29(2):133–142
Vandyke K, Dewar AL, Diamond P, Fitter S, Schultz CG, Sims NA, Zannettino AC (2010) The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J Bone Miner Res 25(8):1759–1770
Araujo JC, Poblenz A, Corn P, Parikh NU, Starbuck MW, Thompson JT, Lee F, Logothetis CJ, Darnay BG (2009) Dasatinib inhibits both osteoclast activation and prostate cancer PC-3-cell-induced osteoclast formation. Cancer Biol Therapy 8(22):2153–2159
Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, Gallick GE, Trudel GC, Paliwal P, Agrawal S et al (2012) Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer 118(1):63–71
Yu EY, Massard C, Gross ME, Carducci MA, Culine S, Hudes G, Posadas EM, Sternberg CN, Wilding G, Trudel GC et al (2011) Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 77(5):1166–1171
Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, Morris MJ, Hudes G, Calabro F, Cheng S et al (2009) Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res 15(23):7421–7428
Twardowski PW, Beumer JH, Chen CS, Kraft AS, Chatta GS, Mitsuhashi M, Ye W, Christner SM, Lilly MB (2013) A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anticancer Drugs 24(7):743–753
Araujo JC TG, Saad F, Armstrong AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev V, Efstathiou E, Oudard S, Morris MJ, Sizer B, Goebell PJ, De Bono JS, Paliwal P, Durham S, Cheng S, Logothetis C (2013) Overall survival (OS) and safety of dasatinib/docetaxel versus docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC): Results from the randomized phase III READY trial. In: ASCO Annual Meeting: 2013; Chicago, IL
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol Off J Am Soc Clin Oncol 26(7):1148–1159
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ (1997) Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 50(6):920–928
Melzack R (1975) The McGill pain questionnaire: major properties and scoring methods. Pain 1(3):277–299
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock AI (2008) Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clinical Cancer Res Off J Am Assoc Cancer Res 14(9):2763–2767
Luan X, Gao C, Zhang N, Chen Y, Sun Q, Tan C, Liu H, Jin Y, Jiang Y (2011) Exploration of acridine scaffold as a potentially interesting scaffold for discovering novel multi-target VEGFR-2 and Src kinase inhibitors. Bioorg Med Chem 19(11):3312–3319
Mezquita B, Mezquita J, Pau M, Mezquita C (2010) A novel intracellular isoform of VEGFR-1 activates Src and promotes cell invasion in MDA-MB-231 breast cancer cells. J Cell Biochem 110(3):732–742
Gavard J, Patel V, Gutkind JS (2008) Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14(1):25–36
Jürgensmeier JM KJ, Odedra R, Logie A, Wood P, Valentine P, Barnett S, Wilkinson RW, Ogilvie DJ, Elvin P, Smith P, Ryan A, Wedge SR (2010) Cediranib alone and in combination with mechanistically distinct antitumor therapies in vivo. In: AACR 101st Annual Meeting: 2010; Washington: Abstract 1372
Tannock IF, Fizazi K, Ivanov S, Karlsson CT, Flechon A, Skoneczna I, Orlandi F, Gravis G, Matveev V, Bavbek S et al (2013) Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol 14(8):760–768
Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C, Albiges L, Attard G, Fizazi K, De Bono JS et al (2013) Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 24(7):1807–1812
AstraZeneca (2011) Investigator’s Brochure, Cediranib, AZD2171, RECENTIN. In. Edited by AstraZeneca
Mendiratta P, Mostaghel E, Guinney J, Tewari AK, Porrello A, Barry WT, Nelson PS, Febbo PG (2009) Genomic strategy for targeting therapy in castration-resistant prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol 27(12):2022–2029
Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M, Weinstein I, Reuveni H, Ben-Sasson SA (2004) Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res 64(3):1058–1066
Vandyke K, Dewar AL, Farrugia AN, Fitter S, Bik To L, Hughes TP, Zannettino AC (2009) Therapeutic concentrations of dasatinib inhibit in vitro osteoclastogenesis. Leukemia Off J Leukemia Soc Am Leukemia Res Fund UK 23(5):994–997
Lee YC, Huang CF, Murshed M, Chu K, Araujo JC, Ye X, de Crombrugghe B, Yu-Lee LY, Gallick GE, Lin SH (2010) Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene 29(22):3196–3207
Id Boufker H, Lagneaux L, Najar M, Piccart M, Ghanem G, Body JJ, Journe F (2010) The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer 10:298
Araujo JC, Trudel GC, Paliwal P (2013) Long-term use of dasatinib in patients with metastatic castration-resistant prostate cancer after receiving the combination of dasatinib and docetaxel. Cancer Manag Res 6:25–30
Garcia-Martin A, Acitores A, Maycas M, Villanueva-Penacarrillo ML, Esbrit P (2013) Src kinases mediate VEGFR2 transactivation by the osteostatin domain of PTHrP to modulate osteoblastic function. J Cell Biochem 114(6):1404–1413
Garcia-Gomez A, Ocio EM, Crusoe E, Santamaria C, Hernandez-Campo P, Blanco JF, Sanchez-Guijo FM, Hernandez-Iglesias T, Brinon JG, Fisac-Herrero RM et al (2012) Dasatinib as a bone-modifying agent: anabolic and anti-resorptive effects. PLoS One 7(4):e34914
Yin JJ, Zhang L, Munasinghe J, Linnoila RI, Kelly K (2010) Cediranib/AZD2171 inhibits bone and brain metastasis in a preclinical model of advanced prostate cancer. Cancer Res 70(21):8662–8673
Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E et al (2012) Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol Off J Am Soc Clin Oncol 30(13):1534–1540
FDA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf. 2011
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307
Kanda S, Miyata Y, Kanetake H, Smithgall TE (2007) Non-receptor protein-tyrosine kinases as molecular targets for antiangiogenic therapy (Review). Int J Mol Med 20(1):113–121
Kilarski WW, Jura N, Gerwins P (2003) Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp Cell Res 291(1):70–82
Yeh M, Gharavi NM, Choi J, Hsieh X, Reed E, Mouillesseaux KP, Cole AL, Reddy ST, Berliner JA (2004) Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem 279(29):30175–30181
Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M (2007) Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell 18(12):5014–5023
Werdich XQ, Penn JS (2005) Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis 8(4):315–326
Acknowledgments
This study (PJC-002/CTEP 8476), supported by the US National Cancer Institute, is led by the Princess Margaret Hospital Phase I Consortium (U01-CA132123), with participation from the University of Chicago Phase II Consortium (N01-CM-2011-00071) and the Johns Hopkins Kimmel Cancer Center Phase I Consortium (U01-CA70095).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1603 kb)
Rights and permissions
About this article
Cite this article
Spreafico, A., Chi, K.N., Sridhar, S.S. et al. A randomized phase II study of cediranib alone versus cediranib in combination with dasatinib in docetaxel resistant, castration resistant prostate cancer patients. Invest New Drugs 32, 1005–1016 (2014). https://doi.org/10.1007/s10637-014-0106-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0106-5