Summary
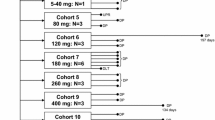
Purpose This study investigated the maximum-tolerated dose (MTD), dose-limiting toxicity (DLT), and pharmacokinetic (PK) profiles of DHP107, a novel oral paclitaxel containing neither Cremophor EL nor P-glycoprotein (P-gp) inhibitor. Patients and methods Patients with advanced solid tumors refractory to all standard treatments were administered a single oral dose of DHP107 on a dose-escalating schedule (60–600 mg/m2) during the first chemotherapy cycle, and intravenous paclitaxel 175 mg/m2 during subsequent cycles. Cohorts of 3 patients were treated at each dose level provided no DLTs were observed. The pharmacokinetics of paclitaxel and its metabolites were investigated for oral DHP107 and intravenous paclitaxel. Results Thirty-four patients were enrolled. Dose-limiting toxicities were not observed, even at the highest dose level (600 mg/m2). Further dose escalation was not performed because pharmacokinetics did not increase proportionally at doses above 250 mg/m2. The coefficient of variance of AUClast DHP107 ranged from 11.8 % to 34.0 %, comparable to 24.4 % of intravenous paclitaxel 175 mg/m2. There were no grade 4 toxicities, whereas grade 3 toxicities included diarrhea (12.1 %), neutropenia (6.1 %) and fatigue (3.0 %). While no objective responses were observed, 11 patients (33.3 %) showed stable disease. Conclusions DHP107 was safe and feasible in patients with advanced malignancies. As exposure of paclitaxel plateau among patients receiving more than 250 mg/m2 of DHP107, the dose escalation of DHP107 may be limited to 250 mg/m2 in further clinical trials.

Similar content being viewed by others
References
Vergote I, Trope CG, Amant F et al (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–53
Kang HJ, Chang HM, Kim TW et al (2008) A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer. Br J Cancer 98:316–22
Di Leo A, Gomez HL, Aziz Z et al (2008) Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 26:5544–52
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–50
Weiss RB, Donehower RC, Wiernik PH et al (1990) Hypersensitivity reactions from taxol. J Clin Oncol 8:1263–8
Liebmann J, Cook JA, Mitchell JB (1993) Cremophor EL, solvent for paclitaxel, and toxicity. Lancet 342:1428
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–36
Zhang M, Tao W, Pan S et al (2009) Low-dose metronomic chemotherapy of paclitaxel synergizes with cetuximab to suppress human colon cancer xenografts. Anticancer Drugs 20:355–63
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–9
Malingre MM, Beijnen JH, Rosing H et al (2001) Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer 84:42–7
Malingre MM, Beijnen JH, Rosing H et al (2001) A phase I and pharmacokinetic study of bi-daily dosing of oral paclitaxel in combination with cyclosporin A. Canc Chemother Pharmacol 47:347–54
Hong JW, Lee IH, Kwak YH et al (2007) Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther 6:3239–47
Shin BS, Kim HJ, Hong SH et al (2009) Enhanced absorption and tissue distribution of paclitaxel following oral administration of DHP 107, a novel mucoadhesive lipid dosage form. Canc Chemother Pharmacol 64:87–94
Britten CD, Baker SD, Denis LJ et al (2000) Oral paclitaxel and concurrent cyclosporin A: targeting clinically relevant systemic exposure to paclitaxel. Clin Cancer Res 6:3459–68
Veltkamp SA, Alderden-Los C, Sharma A et al (2007) A pharmacokinetic and safety study of a novel polymeric paclitaxel formulation for oral application. Canc Chemother Pharmacol 59:43–50
Veltkamp SA, Rosing H, Huitema AD et al (2007) Novel paclitaxel formulations for oral application: a phase I pharmacokinetic study in patients with solid tumours. Cancer Chemother Pharmacol 60:635–42
Malingre MM, Terwogt JM, Beijnen JH et al (2000) Phase I and pharmacokinetic study of oral paclitaxel. J Clin Oncol 18:2468–75
Bardelmeijer HA, Ouwehand M, Malingre MM et al (2002) Entrapment by Cremophor EL decreases the absorption of paclitaxel from the gut. Canc Chemother Pharmacol 49:119–25
Kantarjian H, Giles F, Wunderle L et al (2006) Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 354:2542–51
Rahman A, Korzekwa KR, Grogan J et al (1994) Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2 C8. Cancer Res 54:5543–6
Sparreboom A, Huizing MT, Boesen JJ et al (1995) Isolation, purification, and biological activity of mono- and dihydroxylated paclitaxel metabolites from human feces. Canc Chemother Pharmacol 36:299–304
Chu Z, Chen JS, Liau CT et al (2008) Oral bioavailability of a novel paclitaxel formulation (Genetaxyl) administered with cyclosporin A in cancer patients. Anticancer Drugs 19:275–81
Acknowledgment
This study was supported by a grant from DAE HWA Pharmaceutical Co. and Gangwon Leading Industry Office by Korean government
Conflict of Interest
Hyeyoun Kim works for DAE HWA Pharmaceutical Co., Ltd. as a commissioner, and has a leadership position to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong Sang Hong and Kyu-pyo Kim are equally contributed to this work.
Rights and permissions
About this article
Cite this article
Hong, Y.S., Kim, Kp., Lim, HS. et al. A phase I study of DHP107, a mucoadhesive lipid form of oral paclitaxel, in patients with advanced solid tumors: Crossover comparisons with intravenous paclitaxel. Invest New Drugs 31, 616–622 (2013). https://doi.org/10.1007/s10637-012-9841-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9841-7