Summary
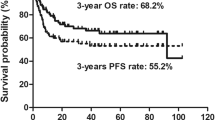
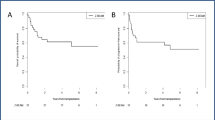
Background Radiolabelled immunotherapy agents have an increasingly significant role in autologous stem cell transplantation (ASCT) by improving the tolerability and increasing the efficacy of the conditioning regimen, thereby reducing the relapse risk. We evaluated the efficacy and safety of yttrium-90-ibritumomab tiuxetan (90Y-ibritumomab) combined with intravenous busulfan, cyclophosphamide, and etoposide (Bu/Cy/E) followed by ASCT in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma (NHL). Methods Each patient received a single dose of 90Y-ibritumomab (0.4 mCi/kg on day −14) with Bu/Cy/E as a conditioning regimen. Results The patient cohort consisted of 19 individuals (ten males), of median age 51 years (range, 25–63 years). Sixteen patients had received two or more chemotherapy regimens before transplantation. Histologies were diffuse large B-cell (n = 14), follicular (n = 2), mantle cell (n = 2), and Burkitt lymphoma (n = 1). All patients engrafted. The median time to neutrophil engraftment was 10 days and time to platelet engraftment was 10 days. Nineteen patients were evaluable for response. The objective overall response rate was 84.2% (16/19): continued CR, 36.8% (7/19); induced CR, 36.8% (7/19); and PR, 10.5% (2/19). With a median follow-up of 29.4 months (13.4–36.6), the estimated 3-year overall survival and event-free survival rates were 52.6% (95% confidence interval [CI] 45.8–59.4) and 26.3% (95% CI 19.8–32.8), respectively. Adverse events were similar to those seen historically with Bu/Cy/E alone, and there were no treatment related deaths. Conclusion In conclusion, 90Y-ibritumomab with Bu/Cy/E and ASCT is feasible in patients with relapsed or refractory B-cell NHL, without increased toxicity.


Similar content being viewed by others
References
Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, Colombat P, Goldstone AH, Gorin NC, Flesh M (1987) High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med 316:1493–1498
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242
Flinn IW, O’Donnell PV, Goodrich A, Vogelsang G, Abrams R, Noga S, Marcellus D, Borowitz M, Jones R, Ambinder RF (2000) Immunotherapy with rituximab during peripheral blood stem cell transplantation for non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant 6:628–632
Flohr T, Hess G, Kolbe K, Gamm H, Nolte H, Stanislawski T, Huber C, Derigs HG (2002) Rituximab in vivo purging is safe and effective in combination with CD34-positive selected autologous stem cell transplantation for salvage therapy in B-NHL. Bone Marrow Transplant 29:769–775
Khouri IF, Saliba RM, Hosing C, Okoroji GJ, Acholonu S, Anderlini P, Couriel D, De Lima M, Donato ML, Fayad L, Giralt S, Jones R, Korbling M, Maadani F, Manning JT, Pro B, Shpall E, Younes A, McLaughlin P, Champlin RE (2005) Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin’s lymphomas. J Clin Oncol 23:2240–2247
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333:1540–1545
Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, Pavlovsky S, Keating A, Yanes B, van Besien K, Armitage JO, Horowitz MM (2001) Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 19:406–413
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ, Jones RB, Tarantolo S, Hu WW, Blume KG, Forman SJ, Champlin RE (2002) Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 8:145–154
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, Blume K, Niland J, Palmer JM, Vaughan W, Fernandez H, Champlin R, Forman S, Andersson BS (2002) Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 8:493–500
Copelan EA, Penza SL, Pohlman B, Avalos BR, Goormastic M, Andresen SW, Kalaycio M, Bechtel TP, Scholl MD, Elder PJ, Ezzone SA, O’Donnell LC, Tighe MB, Risley GL, Young DC, Bolwell BJ (2000) Autotransplantation following busulfan, etoposide and cyclophosphamide in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant 25:1243–1248
Hanel M, Kroger N, Sonnenberg S, Bornhauser M, Kruger W, Kroschinsky F, Hanel A, Metzner B, Birkmann J, Schmid B, Hoffknecht MM, Fiedler F, Ehninger G, Zander AR (2002) Busulfan, cyclophosphamide, and etoposide as high-dose conditioning regimen in patients with malignant lymphoma. Ann Hematol 81:96–102
Kim JG, Sohn SK, Chae YS, Yang DH, Lee JJ, Kim HJ, Shin HJ, Jung JS, Kim WS, Kim DH, Suh C, Kim SJ, Eom HS, Bae SH (2007) Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant 40:919–924
Cheson BD (2003) Radioimmunotherapy of non-Hodgkin lymphomas. Blood 101:391–398
Ferrucci PF, Vanazzi A, Grana CM, Cremonesi M, Bartolomei M, Chinol M, Ferrari M, Radice D, Papi S, Martinelli G, Paganelli G (2007) High activity 90Y-ibritumomab tiuxetan (Zevalin) with peripheral blood progenitor cells support in patients with refractory/resistant B-cell non-Hodgkin lymphomas. Br J Haematol 139:590–599
Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, Kwok C, Yamauchi D, Anderson AL, Falk P, Krishnan A, Kirschbaum M, Kogut N, Nakamura R, O’Donnell M, Parker P, Popplewell L, Pullarkat V, Rodriguez R, Sahebi F, Smith E, Snyder D, Stein A, Spielberger R, Zain J, White C, Raubitschek A (2005) A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood 106:2896–2902
Crawford LM Jr (2002) From the food and drug administration. JAMA 287:1640
Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D, Rodriguez R, Spielberger RT, Falk P, Palmer JM, Forman SJ (2008) Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol 26:90–95
Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R, Avigdor A, Ben-Bassat I, Nagler A (2007) Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin’s lymphoma. Exp Hematol 35:534–540
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-Lopez AJ, Multani P, White CA (2002) Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 20:2453–2463
Cheson BD (2005) The role of radioimmunotherapy with yttrium-90 ibritumomab tiuxetan in the treatment of non-Hodgkin lymphoma. BioDrugs 19:309–322
Cheson BD (2006) Radioimmunotherapy of non-Hodgkin’s lymphomas. Curr Drug Targets 7:1293–1300
Cilley J, Winter JN (2006) Radioimmunotherapy and autologous stem cell transplantation for the treatment of B-cell lymphomas. Haematologica 91:114–120
Vose JM, Bierman PJ, Loberiza FR Jr, Bociek RG, Matso D, Armitage JO (2007) Phase I trial of (90)Y-ibritumomab tiuxetan in patients with relapsed B-cell non-Hodgkin’s lymphoma following high-dose chemotherapy and autologous stem cell transplantation. Leuk Lymphoma 48:683–690
Fernandez HF, Escalon MP, Pereira D, Lazarus HM (2007) Autotransplant conditioning regimens for aggressive lymphoma: are we on the right road? Bone Marrow Transplant 40:505–513
Press OW, Eary JF, Gooley T, Gopal AK, Liu S, Rajendran JG, Maloney DG, Petersdorf S, Bush SA, Durack LD, Martin PJ, Fisher DR, Wood B, Borrow JW, Porter B, Smith JP, Matthews DC, Appelbaum FR, Bernstein ID (2000) A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood 96:2934–2942
de Kreuk M, Ossenkoppele GJ, Meijer CJ, Huijgens PC (1996) Prognostic factors for survival of non-Hodgkin’s lymphoma patients treated with high-dose chemotherapy and autologous bone marrow transplantation. Bone Marrow Transplant 17:963–971
Guglielmi C, Gomez F, Philip T, Hagenbeek A, Martelli M, Sebban C, Milpied N, Bron D, Cahn JY, Somers R, Sonneveld P, Gisselbrecht C, Van Der Lelie H, Chauvin F (1998) Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol 16:3264–3269
Kunala S, Macklis RM (2001) Ionizing radiation induces CD20 surface expression on human B cells. Int J Cancer 96:178–181
Hernandez MC, Knox SJ (2004) Radiobiology of radioimmunotherapy: targeting CD20 B-cell antigen in non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys 59:1274–1287
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, Cripe L, Wiseman G, Olejnik T, Multani PS, White CA (2002) Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol 20:3262–3269
Acknowledgment
We thank Bayer Korea Ltd. (Seoul, Korea), which provided the 90Y-ibritumomab tiuxetan. All authors reviewed and approved the paper. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, B.W., Kim, W.S., Kim, C. et al. Yttrium-90-ibritumomab tiuxetan in combination with intravenous busulfan, cyclophosphamide, and etoposide followed by autologous stem cell transplantation in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Invest New Drugs 28, 516–522 (2010). https://doi.org/10.1007/s10637-009-9283-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9283-z