Abstract
Background
Histopathologic differentiation between the stages of Barrett’s carcinogenesis is often challenging. Liver–intestine (LI)-cadherin, an intestine-specific marker, is involved in intestinal metaplasia development in gastric and colon cancers and could be of value in diagnosis and differentiation.
Aims
To examine the expression of LI-cadherin in the sequence of Barrett’s carcinogenesis and to evaluate its association with clinicopathological data.
Methods
LI-cadherin expression was immunohistologically investigated, by use of anti-CDH17 antibody, in gastric mucosa (GM) biopsies taken from the cardia (n = 9), in Barrett’s esophagus (BE) without intraepithelial neoplasia (without IEN) (n = 9) and BE with low-grade IEN (n = 11), and in esophageal adenocarcinoma (ADC) (n = 13).
Results
The immunoreactivity score was highest in adenocarcinoma (mean IRS = 4.0), and dropped gradually from BE with IEN and BE without IEN (mean IRS = 2.0) to cardia mucosa (IRS = 0). Similarly, the intensity of staining and the percentage of positive cells increased during the sequential stages of BE carcinogenesis. Comparative analysis showed that LI-cadherin expression was significantly different between cardiac epithelium and ADC. Also, percentage of positive cells in GM was significantly different from that in BE with IEN. LI-cadherin IRS was lower for tumors with poor differentiation than for moderately differentiated tumors, but the difference was not statistically significant.
Conclusions
LI-cadherin is a sensitive marker of intestinal metaplasia and can be helpful for early histologic diagnosis of Barrett’s esophagus; it is, however, not significantly different between BE with and without IEN, and cannot be used to distinguish between these.
Similar content being viewed by others
Introduction
Although the stages of esophageal carcinogenesis from Barrett’s esophagus to adenocarcinoma are known, the outcome of surveillance programs is not satisfactory. The rapidly rising incidence of esophageal adenocarcinoma over the past two decades has led to trials to identify patients with Barrett’s esophagus (BE) and patients with a high risk of progression to adenocarcinoma (ADC) [1, 2].
The presence of Barrett’s esophagus with intestinal metaplasia is used as a marker for identification of patients in need of endoscopic surveillance. However, the best predictor of future development of adenocarcinoma is diagnosis of dysplasia (intra-epithelial neoplasia). Unfortunately, assessment of dysplasia may be difficult, and interobserver variability among pathologists is still high [3].
The current definition of Barrett’s esophagus requires the presence of specialized columnar epithelium with goblet cells [4, 5]. The value of this assessment could be diminished because of patchy distribution of goblet cells within the columnar-lined esophagus. Thus, in several countries, a new definition has been proposed [6, 7]. Several authors have suggested that gastric metaplasia in the esophagus without goblet cells are also at risk of malignant transformation [8, 9]. Reliable immunohistochemical markers are needed to determine intestinal differentiation in the absence of goblet cells.
It is, furthermore, important to predict tumor aggressiveness or potential lymph node metastasis preoperatively, because esophageal adenocarcinoma is still a cancer with poor prognosis. There is a need for markers helpful in preoperative assessment of the possible outcome of medical intervention.
Cadherins are transmembrane glycoproteins responsible for cell recognition, adhesion, and the strength of interactions between cells [10]. Abnormalities in adhesion are the most important factors in generating invasive cancer cells [11, 12]. Moreover, these traits of cancer cells correlate with low expression of specific cadherins. The loss of intercellular contact which results from reduction of the amount of cadherin creates favorable conditions for migration of invasive cancer cells [13, 14]. Classical cadherin (i.e. the N, E, and P-forms) adherens junctions have a variety of functions in cell adhesion. Reduced expression of classical cadherin is observed in several tumor cell lines and correlates with the invasiveness of the tumor [13, 14]. Liver–intestine (LI)-cadherin is a member of the cadherin superfamily, although it contains seven rather than five molecular domains [15, 16]. It is expressed by enterocytes and goblet cells in the intestine, but not in the upper gastrointestinal tract. Abnormalities in LI-cadherin expression have been shown to serve as marker for early detection and changes toward development of gastric intestinal metaplasia and well-differentiated gastric adenocarcinomas [17], and as a marker of other carcinomas [18, 19].
LI-cadherin is one of the transcriptional targets of CDX2 (the caudal-type homeobox transcription factor) which proved to be important during early differentiation and maintenance of intestinal epithelium [20]. The role of CDX in Barrett’s carcinogenesis is well known [21, 22], thus LI-cadherin may serve as a marker of the connection with CDX2 and could be of value in diagnosis of BE.
In this study, we examined, by immunohistochemistry, expression of LI-cadherin in gastric metaplastic tissues, in BE with and without intraepithelial neoplasia (IEN), and in esophageal adenocarcinoma (ADC) tissues. We also evaluated the role of LI-cadherin in Barrett-related carcinogenesis by analyzing associations between LI-cadherin and clinicopathological data for ADC patients.
Methods
Patients and Samples
The study group comprised 42 patients (10 women and 32 men); mean patient age was 58.9 years (minimum 45 years, maximum 74 years). Esophageal biopsies were obtained for 29 patients and surgical esophageal specimens for 13. Formalin-fixed, paraffin-embedded tissue samples were selected from the archive, and hematoxylin and eosin (H&E)-stained slides were reviewed to verify initial diagnoses and to select suitable areas for immunohistochemical staining. Samples with cardiac-like mucosa (n = 9), from Barrett’s esophagus without intraepithelial neoplasia (IEN) (n = 9) and with low-grade IEN (n = 11), and from esophageal adenocarcinoma (n = 13) were studied. Participants in the study were patients whose routine samples had been examined at the Institute of pathology, Klinikum Bayreuth, Germany, and the Department of Pathology, Chair of Oncology, Medical University of Lodz, Lodz, Poland, in the years 2010–2011. The study protocol was approved the local Ethics Committee (no. RNN/9/09/KE).
Immunohistochemistry was performed automatically by use of a BenchMark automatic stainer (Roche, Mannheim, Germany). Reagents used for immunohistochemistry (cell conditioning solution, reaction buffer, UV red enhancing system) were all obtained from Roche.
Commercially available antibody against CDH17 (Sigma–Aldrich, St Louis, USA) was diluted 1:600 before use.
Stained slides were coated using Eukitt as mounting medium (Struers, Willich, Germany).
All stained slides were evaluated by use of a standard light microscope (BH-2; Olympus, Hamburg, Germany) at 40, 100, 200, and 400 magnification. The staining intensity was graded semiquantitatively as 0 (absent), 1 (weak), 2 (moderate), or 3 (strong). Also, the number of positively stained cells was estimated as a percentage of all epithelial cells of the target lesion. By analogy with Remmele and Stegner [23], an immunoreactivity score (IRS) was calculated as the product of points for staining intensity (see above) and percentage of positive cells as follows: 0 % (0), <10 % (1), 11–50 % (2), 51–80 % (3), and 81–100 % (4), the IRS score ranging from 0 to 12.
Photographs were taken by using hardware (AX-70 light microscope, Imaging Solutions camera) and software (Cell F imaging software) from Olympus and Olympus Soft Imaging Solutions (Mannheim, Germany), respectively.
Statistical Analysis
The results were analyzed by use of well known statistical methods, by using StatSoft (Tulsa, USA) Statistica for Windows, release 8.0. Variables with a heavily skewed distribution were compared between groups by use of the Kruskal–Wallis test followed by post-hoc comparisons with Dunn’s test. Test for proportions with Bonferroni correction was used to analyze differences between discrete variables. Survival analysis was used to assess the relationship between survival time and one of independent variables in a Cox regression model. A P value <0.05 (two-tailed) was regarded as statistically significant.
Results
In cardiac epithelium without pathological changes no staining was observed whereas all other lesions had areas of weak, moderate, or strong staining. LI-cadherin was accentuated at the luminal side of the epithelium. By use of the staining techniques described, LI-cadherin is revealed as brown membranous staining (Figs. 1, 2).
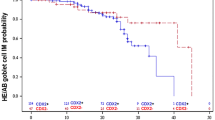
In cases of more advanced neoplasia the IRS, staining intensity, and percentage of positive cells increased significantly. The immunoreactivity score was highest in adenocarcinoma (range 0–7.5, mean = 4), it dropped gradually from Barrett’s with IEN (range 0–2, mean = 2) via BE without IEN (range 0–2, mean = 2) to cardiac mucosa (IRS = 0) (Fig. 1).
Similarly, the intensity of staining and the percentage of positive cells increased during the sequential stages of BE carcinogenesis (Fig. 1).
Comparative analysis showed that percentage of LI-cadherin-positive cells, staining intensity, and IRS were significantly different between cardiac epithelium and ADC (Table 1). Also, percentage of positive cells for GM was significantly different from that for BE with IEN (Table 1).
LI-cadherin expression was not significantly different between Barrett’s mucosa with and without EIN, or between BE mucosa and adenocarcinoma (Fig. 1; Table 1).
However, we found that LI-cadherin expression in moderately-differentiated ADC (G2) was much higher than in BE with IEN (Fig. 1), although the difference did not reach statistical significance. For tumors with poor differentiation LI-cadherin IRS was lower than in moderately differentiated tumors but the difference was not significant (Fig. 2).
LI-cadherin immunoreactivity was detected in 8 of 13 (61.5 %) esophageal cancerous tissues.
Immunoreactivity score increased, but not significantly, with the tumor stage: mean IRS in T2 N1 = 3.2; IRS in T2N2 = 6.5; IRS in T3N1 = 5.4. No significant correlations were found between IRS and T or between IRS and N (Fig. 2).
Furthermore, LI-cadherin expression was not significantly related to survival time (Fig. 3).
However, we observed tendency to lower survival of patients with high LI-cadherin expression. Seven patients with two-year survival had low mean IRS (range 0–12, mean = 3.6; 4 patients with IRS = 0). In contrast, six patients with survival less than two years had high mean IRS (range 0–9, mean = 6), with only one patients with IRS = 0 (Fig. 4).
Discussion
This study confirmed that LI-cadherin expression differed significantly between proximal gastric epithelium (cardia) and lesions in the BE–carcinogenesis sequence. It is helpful in distinguishing between cardia epithelium and BE mucosa with dysplasia.
However, no significant differences could be found between Barrett’s mucosa with and without IEN. Thus, LI-cadherin could not be helpful in indicating the presence of dysplasia. Similar results were obtained by Weiman and co-workers [24, 25], who also found LI-cadherin did not differ between low-grade and high-grade dysplasia.
In the Weiman et al. study, LI-cadherin expression in invasive adenocarcinoma was weaker than in BE with HG-IEN. The authors explained this phenomenon on the basis that cancer cells become less differentiated during disease progression, resulting in a downregulation of LI-cadherin, and concluded that strong staining reaction may confirm a diagnosis of dysplasia, and that abrupt loss of immunoreactivity could be indicative of areas where invasion is beginning [25].
Weiman et al. did not reveal the grading of their adenocarcinoma cases. It is possible they were mostly poorly differentiated, because our study revealed greater LI-cadherin immunoreactivity in biopsies from well-differentiated esophageal adenocarcinoma tissues than in those from poorly differentiated adenocarcinoma tissues. Different LI-cadherin expression between G2 and G3 adenocarcinoma was not statistically significant, probably because of the small groups of patients.
Studies of other cancers have shown that LI-cadherin expression was strong in well-differentiated carcinoma cases, whereas it is expressed less or not at all in less differentiated areas and poorly differentiated carcinoma cases [19, 26].
Dong et al. studied LI-cadherin expression in gastric cancer and in intestinal metaplasia in its precancerous condition [27]. LI-cadherin was absent from normal gastric tissue, which is similar to our findings. The highest expression of LI-cadherin protein and LI-cadherin mRNA level were observed in intestinal metaplasia, compared with gastric cancer tissues. Furthermore, LI-cadherin expression decreased with lower cancer differentiation grade—it was higher in well differentiated gastric cancerous tissue.
We have assessed for the first time the association of LI-cadherin expression with clinicopathological data for esophageal adenocarcinoma.
We have shown that LI-cadherin immunoreactivity in esophageal adenocarcinoma has similar association with clinicopathological data as reported previously for other carcinomas [27, 28]. In our study LI-cadherin expression was higher in advanced adenocarcinomas assessed by TNM classification, however differences were not significant.
These results suggest that patients with high levels of LI-cadherin expression could have a poorer prognosis than patients with low LI-cadherin expression.
Thus, we assessed, for the first time, the real survival time for patients with esophageal adenocarcinoma. There was a tendency to worse survival of patients with higher LI-cadherin immunoreactivity, although the difference was not statistically significant.
Similar to our results, Dong et al. found higher expression of LI-cadherin in the presence of lymph node metastases [27]. Similar to our findings, the highest LI-cadherin expression was found in stage TNM III but, both in our study and in that of Dong et al., differences between LI-cadherin expression in comparison with TNM staging were not statistically significant. The authors concluded that greater expression of LI-cadherin occurs in the final stage of gastric cancer. We cannot agree with this hypothesis, because we found LI-cadherin staining in earlier stages of BE carcinogenesis.
In contrast with these findings, a study of human colorectal cancer showed that reduced LI-cadherin expression was significantly associated with high tumor grade, lymphatic invasion, lymph node metastasis, and advanced pTNM stage. Takamura et al. suggested that analysis of reduced LI-cadherin expression may help to indicate the biological aggressiveness of malignancy [26].
This is in concordance with the known function of the other cadherins which are not only involved in adhesion between cells but also inhibit tumor growth. Lack of cadherin is one of the factors that may induce metastasis of cancer cells, probably by transduction of signaling pathways and factors that activate tumor cells to invade adjacent cells and tissues. The data indicate that reduced expression of cadherin can be a marker of breast, prostate, colon, and stomach cancer cells [29, 30]. Results for LI-cadherin, which could be very different from those for others cadherin, and their correlation with tumor advance are controversial.
Several studies have been performed to establish the role of LI-cadherin, particularly in gastric carcinogenesis. Ko et al. detected overexpression and colocalization of CDX2 and LI-cadherin in gastric intestinal metaplasia and adenocarcinoma, and suggested that aberrant upregulation of CDX2 and, consequently, activation of intestinal genes may be one possible mechanism of induction of intestinal metaplasia [15, 16, 28, 31]. Ko et al. observed a strong association between LI-catherin and CDX2 expression and the aggressiveness of gastric carcinoma [28]. CDX2 expression has been demonstrated in Barrett’s metaplasia [32].
Takamura et al. [19] found that the interaction between LI-cadherin and galectin-3 is mediated by the carbohydrate recognition domain, suggesting that galectin-3 binds to LI-cadherin on the cell surface of pancreatic carcinoma. Dong et al. [33] also found that LI-cadherin mRNA expression levels inversely correlated with the amount of galectin-3 mRNA in gastric cancer tissue. These results suggest that LI-cadherin and galectin-3 may have different roles in the development of gastric cancer. The authors hypothesized that expression of LI-cadherin could be hindered by galectin-3 during the course of gastric cancer. However, the real mechanism of galectin-3 regulation of LI-cadherin is still unknown.
Further investigations are required to find the mechanism of action of LI-cadherin in esophageal adenocarcinoma.
In summary, LI-cadherin is a sensitive marker of intestinal differentiation and early esophageal malignancy. We have shown, for the first time, lower LI-cadherin expression in poorly differentiated esophageal adenocarcinoma, which could be caused by loss of differentiation of ADC cells and loss of the ability to produce goblet cells in advanced cases. We have also assessed, again for the first time, LI-expression in relation to survival of esophageal adenocarcinoma patients and we found a tendency to worse survival of patients with higher LI-cadherin immunoreactivity.
The limitation of the study is the small groups of cases studied. Increasing the number of cases may help achieve statistical significance for some categories, particularly between different ADC grades and TNM stages.
References
Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9.
Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin North Am. 2002;11:235–256.
Kerkhof M, van Dekken H, Steyerberg EW, et al. CYBAR study group. Grading of dysplasia in Barrett’s oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology. 2007;50:920–927.
Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–1032.
Wang KK, Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797.
Playford RJ. New British society of gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006;55:442.
Takubo K, Vieth M, Aida J, et al. Differences in the definitions used for esophageal and gastric diseases in different countries: endoscopic definition of the esophagogastric junction, the precursor of Barrett’s adenocarcinoma, the definition of Barrett’s esophagus, and histologic criteria for mucosal adenocarcinoma or high-grade dysplasia. Digestion. 2009;80:248–257.
Takubo K, Aida J, Naomoto Y, et al. Cardiac rather than intestinal type background in endoscopic specimens of minute Barrett adenocarcinoma. Hum Pathol. 2009;40:65–74.
Liu W, Hahn H, Odze RD, et al. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol. 2009;104:816–824.
Ranscht B. Cadherins and catenins: interactions and functions in embryogenic development. Curr Opin Cell Biol. 1994;6:740–746.
Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627.
Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339.
Okegawa T, Pong RC, Li Y, et al. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51:445–457.
Cavallaro U, Christofori G. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Act. 2001;1552:39–45.
Berndorff D, Gessner R, Kreft B, et al. Liver-intestine cadherin: molecular cloning and characterization of a novel Ca2+-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol. 1994;125:1353–1369.
Gessner R, Tauber R. Intestinal cell adhesion molecules. Liver-intestine cadherin. Ann NY Acad Sci. 2000;915:136–143.
Grotzinger C, Kneifel J, Patschan D, et al. LI-cadherin: a marker of gastric metaplasia and neoplasia. Gut. 2001;49:73–81.
Wong BW, Luk JM, Ng IO, et al. Identification of liver-intestine cadherin in hepatocellular carcinoma—a potential disease marker. Biochem Biophys Res Commun. 2003;311:618–624.
Takamura M, Sakamoto M, Ino Y, et al. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci. 2003;94:425–430.
Freund JN, Domon-Dell C, Kedinger M, et al. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957–969.
Colleypriest BJ, Farrant JM, Slack JM, et al. The role of Cdx2 in Barrett’s metaplasia. Biochem Soc Trans. 2010;38:364–369.
Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–G218.
Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140.
Weimann A, Zimmermann M, Gross M, et al. CDX2 and LI-cadherin expression in esophageal mucosa: use of both markers can facilitate the histologic diagnosis of Barrett’s esophagus and carcinoma. Int J Surg Pathol. 2010;18:330–337.
Weimann A, Rieger A, Zimmermann M, et al. Comparison of six immunohistochemical markers for the histologic diagnosis of neoplasia in Barrett’s esophagus. Virchows Arch. 2010;457:537–545.
Takamura M, Yamagiwa S, Wakai T, et al. Loss of liver-intestine cadherin in human intrahepatic cholangiocarcinoma promotes angiogenesis by up-regulating metal-responsive transcription factor-1 and placental growth factor. Int J Oncol. 2010;36:245–254.
Dong W, Yu Q, Xu Y. Altered expression of a Li-cadherin in gastric cancer and intestinal metaplasia. Dig Dis Sci. 2007;52:536–542.
Ko S, Chu KM, Luk JM, et al. CDX2 co-localizes with liver-intestine cadherin in intestinal metaplasia and adenocarcinoma of the stomach. J Pathol. 2005;205:615–622.
Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76–85.
Ahmad A, Hart IR. Mechanisms of metastasis. Crit Rev Oncol Hematol. 1997;26:163–173.
Hinoi T, Lucas PC, Kuick R, et al. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology. 2002;123:1565–1577.
Eda A, Osawa H, Satoh K, et al. Aberrant expression of CDX2 in Barrett’s epithelium and inflammatory esophageal mucosa. J Gastroenterol. 2003;38:14–22.
Dong WG, Yu QF, Xu Y, et al. Li-cadherin is inversely correlated with galectin-3 expression in gastric cancer. Dig Dis Sci. 2008;53:1811–1817.
Acknowledgments
The work was supported by the Medical University of Lodz, contract grant numbers 503/1-002-01/503-01 and 502-03/1-002-01/502-14-043.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Anna Mokrowiecka and Sarah Zonnur contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mokrowiecka, A., Zonnur, S., Veits, L. et al. Liver–Intestine-Cadherin Is a Sensitive Marker of Intestinal Differentiation During Barrett’s Carcinogenesis. Dig Dis Sci 58, 699–705 (2013). https://doi.org/10.1007/s10620-012-2425-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2425-8