Abstract
Background and Aims
Studies have shown a decrease in key tight junction (TJ) proteins such as ZO-1 and occludin in both inflammatory bowel disease (IBD) and experimental models of inflammation. Our group has also shown an increase in claudin-1 in experimental colitis.
Methods
IEC-18 cells were treated with increasing doses of tumor necrosis factor alpha (TNFα). The TJ was assessed by transepithelial resistance (TER), permeability, Western blot, PCR, and immunofluorescence. Mucosal samples from patients with ulcerative colitis (UC), Crohn’s disease (CD), and without IBD (normal) were assayed for TJ proteins occludin and claudin-1 by Western blot and a ratio of claudin-1 to occludin (C:O) was calculated.
Results
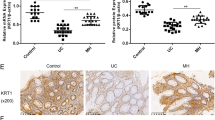
IEC-18 cells had increased permeability, decreased TER and an increase in claudin-1 with TNFα treatment. In human specimens, there was a decrease in occludin and an increase in claudin-1 leading to a significant increase in the C:O ratio in diseased UC colon compared to non-diseased UC colon (P < 0.001) and normal colon (P < 0.01). In CD, the C:O ratio was similar in all CD tissue irrespective of disease status.
Conclusions
Treatment of IEC-18 cells with TNFα, a key inflammatory cytokine in IBD, led to a significant increase in claudin-1 expression. There was a significant increase in the C:O ratio in diseased colon in UC compared to the healthy appearing UC colon and normal controls. The C:O ratio was unchanged in CD despite presence or abscence of gross disease. This suggests that there may be an underlying difference in the TJ between UC and CD.




Similar content being viewed by others
References
MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–305.
Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709.
Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995;101:428–435.
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753.
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363.
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788.
Matter K, Balda MS. Occludin and the functions of tight junctions. Int Rev Cytol. 1999;186:117–146.
Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi:10.1146/annurev.physiol.68.040104.131404.
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550.
Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–G228.
Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH Jr. A freeze fracture study of Crohn’s disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537–547.
Olson TS, Reuter BK, Scott KG, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi:10.1084/jem.20050407.
Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi:10.1016/j.jss.2006.07.050.
Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153–G162. doi:10.1152/ajpgi.00395.2004.
Reuter BK, Pizarro TT. Mechanisms of tight junction dysregulation in the SAMP1/YitFc model of Crohn’s disease-like ileitis. Ann N Y Acad Sci. 2009;1165:301–307. doi:10.1111/j.1749-6632.2009.04035.x.
Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309.
Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–2009.
Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23:S146–S150. doi:10.1111/j.1440-1746.2008.05405.x.
Poritz L, Sundstrom J, Harris L, Barber A, Antonetti D. Alteration of occludin expression in intestinal inflammation. J Surg Res. 2009;151:188.
Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi:10.1152/ajpgi.00173.2003.
Soler AP, Marano CW, Bryans M, et al. Activation of NF-kappaB is necessary for the restoration of the barrier function of an epithelium undergoing TNF-alpha-induced apoptosis. Eur J Cell Biol. 1999;78:56–66.
Schmitz H, Fromm M, Bentzel CJ, et al. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137–146.
Yoo J, Nichols A, Song JC, et al. Bryostatin-1 attenuates TNF-induced epithelial barrier dysfunction: role of novel PKC isozymes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G703–G712. doi:10.1152/ajpgi.00214.2002.
Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC, Jr. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–677.
Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–G1288.
Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849–855.
Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885.
May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–1632.
Peeters M, Geypens B, Claus D, et al. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology. 1997;113:802–807.
Koltun WA, Tilberg AF, Page MJ, Poritz LS. Bowel permeability is improved in Crohn’s disease after ileocolectomy. Dis Colon Rectum. 1998;41:687–690.
Zeissig S, Bojarski C, Buergel N, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi:10.1136/gut.2003.036632.
Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi:10.1136/gut.2006.094375.
Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749–1753.
Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172.
Strater J, Wellisch I, Riedl S, et al. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160–167.
Di Sabatino A, Ciccocioppo R, Cinque B, et al. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut. 2004;53:70–77.
Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology. 2001;121:1145–1157.
ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut. 2002;50:206–211.
Huo Q, Kinugasa T, Wang L, et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res. 2009;29:851–857.
Kinugasa T, Huo Q, Higashi D, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27:3729–3734.
Mees ST, Mennigen R, Spieker T, et al. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int J Colorectal Dis. 2009;24:361–368. doi:10.1007/s00384-009-0653-y.
Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. doi:10.1038/labinvest.2008.78.
Acknowledgments
Presented at Digestive Disease Week, May 2009.
Grant support
NIH: K08 GM081595 (Poritz).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poritz, L.S., Harris, L.R., Kelly, A.A. et al. Increase in the Tight Junction Protein Claudin-1 in Intestinal Inflammation. Dig Dis Sci 56, 2802–2809 (2011). https://doi.org/10.1007/s10620-011-1688-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1688-9