Abstract
Background and Aims
Nonalcoholic steatohepatitis (NASH) is associated with fat accumulation in the liver, and develops to cirrhosis with the progression of hepatic fibrosis. Eicosapentaenoic acid (EPA) is used to treat hyperlipidemia, and suppresses hepatic fat accumulation. As the effect of EPA on NASH remains unclear, we assessed the therapeutic effect of EPA and its mechanisms in an animal model of NASH.
Methods
Wistar rats were fed a methionine- and choline-deficient (MCD) diet for 20 weeks, and given EPA ethyl ester (EPA-E, 1,000 mg/kg/day) or vehicle by gavage from week 12, at which hepatic fibrosis has already established. The liver was histologically analyzed for fibrosis and α-smooth muscle actin (αSMA) expression, and hepatic levels of transforming growth factor-β1 (TGF-β1), fibrogenic gene expression, reactive oxygen species (ROS), and triglyceride (TG) content were determined. Serum oxidative markers were also measured.
Results
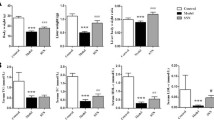
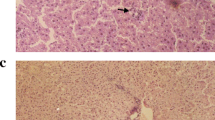
EPA-E treatment significantly suppressed the MCD-induced increase in fibrotic area of liver sections, with repressed macronodule formation. EPA-E also suppressed increases in hepatic fibrogenic factors, αSMA expression, TGF-β1 level, and messenger RNA (mRNA) levels of procollagens and connective tissue growth factor. EPA-E reduced MCD-induced increases in hepatic ROS level, serum oxidative markers, 8-isoprostane and ferritin, and hepatic TG content. Attenuation of hepatic fibrosis by EPA-E was significantly correlated with hepatic ROS level, but not TG content.
Conclusions
EPA-E attenuates progression of hepatic fibrosis in developed steatohepatitis, and this effect is likely mediated by inhibition of ROS production. These actions may elicit the therapeutic effect of EPA-E against NASH.




Similar content being viewed by others
References
Duvnjak M, Lerotić I, Baršić N, Tomašić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539–4550.
Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr. 2006;83(Suppl):1499S–1504S.
Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139.
Tsukamoto H, Rippe R, Niemelä O, Lin M. Roles of oxidative stress in activation of Kupffer and Ito cells in liver fibrogenesis. J Gastroenterol Hepatol. 1995;10(Suppl 1):S50–S53.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218.
Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28.
Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGFα and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468.
Zamara E, Novo E, Marra F, et al. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68.
Svegliati-Baroni G, Saccomanno S, van Goor H, Jansen P, Benedetti A, Moshage H. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver. 2001;21:1–12.
Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56–62.
Garcia-Monzόn C, Martín-Pérez E, Iacono OL, et al. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol. 2000;33:716–724.
Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62.
Haukeland JW, Damås JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174.
Sumida Y, Nakashima T, Yoh T, et al. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32–38.
Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098.
Spadaro L, Magliocco O, Spampinato D, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40:194–199.
Capanni M, Calella F, Biagini MR, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23:1143–1151.
Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot Essent Fatty Acids. 2009;80:229–238.
Berge RK, Madsen L, Vaagenes H, Tronstad KJ, Göttlicher M, Rustan AC. In contrast with docosahexaenoic acid, eicosapentaenoic acid and hypolipidaemic derivatives decrease hepatic synthesis and secretion of triacylglycerol by decreased diacylglycerol acyltransferase activity and stimulation of fatty acid oxidation. Biochem J. 1999;343(Pt 1):191–197.
Clarke SD. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am J Physiol Gastrointest Liver Physiol. 2001;281:G865–G869.
Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Suppression of hepatic fat accumulation by highly purified eicosapentaenoic acid prevents the progression of D-galactosamine-induced hepatitis in mice fed with a high-fat/high-sucrose diet. Biochim Biophys Acta. 2009;1791:281–288.
Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57:451–455.
Armstrong MB, Towle HC. Polyunsaturated fatty acids stimulate hepatic UCP-2 expression via a PPARalpha-mediated pathway. Am J Physiol Endocrinol Metab. 2001;281:E1197–E1204.
Li M, Zhu Q, Hu C, Giesy JP, Kong Z, Cui Y. Protective effects of eicosapentaenoic acid on genotoxicity and oxidative stress of cyclophosphamide in mice. Environ Toxicol. 2010;5 (Epub ahead of print).
Takahashi M, Tsuboyama-Kasaoka N, Nakatani T, et al. Fish oil feeding alters liver gene expressions to defend against PPARα activation and ROS production. Am J Physiol Gastrointest Liver Physiol. 2002;282:G338–G348.
Schneider SM, Fung VS, Palmblad J, Babior BM. Activity of the leukocyte NADPH oxidase in whole neutrophils and cell-free neutrophil preparations stimulated with long-chain polyunsaturated fatty acids. Inflammation. 2001;25:17–23.
Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344.
Bataller R, Schwabe RF, Choi YH, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394.
Demoz A, Willumsen N, Berge RK. Eicosapentaenoic acid at hypotriglyceridemic dose enhances the hepatic antioxidant defense in mice. Lipids. 1992;27:968–971.
Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid ethyl ester prevents development of steatosis and hepatic fibrosis in rats. Dig Dis Sci. 2010;55:631–641.
Takeo S, Nasa Y, Tanonaka K, et al. Effects of long-term treatment with eicosapentaenoic acid on the heart subjected to ischemia/reperfusion and hypoxia/reoxygenation in rats. Mol Cell Biochem. 1998;188:199–208.
Yamada H, Yoshida M, Nakano Y, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2008;28:2173–2179.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr. 2005;135:549–555.
Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–1076.
Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653.
George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39:756–764.
Veteläinen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J Gastroenterol Hepatol. 2007;22:1526–1533.
Kennedy JI Jr, Chandler DB, Fulmer JD, Wert MB, Grizzle WE. Dietary fish oil inhibits bleomycin-induced pulmonary fibrosis in the rat. Exp Lung Res. 1989;15:315–329.
Manoury B, Nenan S, Leclerc O, et al. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11.
Frøyland L, Vaagenes H, Asiedu DK, Garras A, Lie Ø, Berge RK. Chronic administration of eicosapentaenoic acid and docosahexaenoic acid as ethyl esters reduced plasma cholesterol and changed the fatty acid composition in rat blood and organs. Lipids. 1996;31:169–178.
Osmundsen H, Braud H, Beauseigneur F, Gresti J, Tsoko M, Clouet P. Effects of dietary treatment of rats with eicosapentaenoic acid or docosahexaenoic acid on hepatic lipid metabolism. Biochem J. 1998;331:153–160.
Sekiya M, Yahagi N, Matsuzaka T, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539.
Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33–60.
De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-β signaling in activated rat hepatic stellate cells. J Biol Chem. 1999;274:33881–33887.
Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-β1. Lab Invest. 2004;84:1013–1023.
Wang H, Kochevar IE. Involvement of UVB-induced reactive oxygen species in TGF-β biosynthesis and activation in keratinocytes. Free Radic Biol Med. 2005;38:890–897.
Albright CD, Salganik RI, Craciunescu CN, Mar MH, Zeisel SH. Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-β1 in immortalized rat hepatocytes. J Cell Biochem. 2003;89:254–261.
Herrera B, Murillo MM, Álvarez-Barrientos A, Beltrán J, Fernández M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-β in fetal rat hepatocytes. Free Radic Biol Med. 2004;36:16–26.
Kayanoki Y, Fujii J, Suzuki K, Kawata S, Matsuzawa Y, Taniguchi N. Suppression of antioxidative enzyme expression by transforming growth factor-β1 in rat hepatocytes. J Biol Chem. 1994;269:15488–15492.
Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic Biol Med. 2010;48:1–15.
An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol. 2009;297:F895–F903.
Tanaka N, Sano K, Horiuchi A, Tanaka E, Kiyosawa K, Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42:413–418.
Acknowledgments
We thank Ms. Reiko Ono and Mr. Shin-ichi Akimoto for their skillful assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kajikawa, S., Imada, K., Takeuchi, T. et al. Eicosapentaenoic Acid Attenuates Progression of Hepatic Fibrosis with Inhibition of Reactive Oxygen Species Production in Rats Fed Methionine- and Choline-Deficient Diet. Dig Dis Sci 56, 1065–1074 (2011). https://doi.org/10.1007/s10620-010-1400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1400-5