Abstract
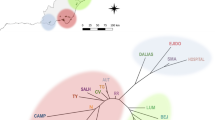
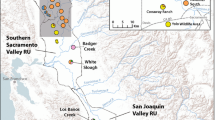
Vernal pool ecosystems are declining throughout California, with only 10% of historic habitat remaining. This has endangered many specialist endemic plant species, leaving extant populations fragmented, isolated, and threatened or endangered. Recovery plans for the increasing number of endangered vernal pool species require information on their genetic and ecological status to guide conservation and restoration efforts. Federally threatened Neostapfia colusana (Colusa grass) and federally endangered Tuctoria greenei (Greene’s tuctoria) are two endemic vernal pool grasses of high conservation concern in central California. Remaining populations are highly fragmented due to range-wide habitat destruction. Using five polymorphic microsatellite markers for each species, we performed genetic surveys of 240 individuals from eight vernal pools for N. colusana, and 317 individuals from 13 vernal pools for T. greenei. We detected high within-population genetic diversity for both species, with average allelic diversities of 24 alleles/locus (mean Hobs = 0.68, mean Hexp = 0.71) for N. colusana, and 19 alleles/locus (mean Hobs = 0.77, and mean Hexp = 0.79) for T. greenei. Bayesian clustering and AMOVA indicated two genetically distinct population groups for N. colusana (Fst = 0.268, P < 0.0001), and three for T. greenei (Fst = 0.11, P < 0.0001). We found very slight temporal genetic structure at one N. colusana (Fst = 0.013, P < 0.05) and two T. greenei (Fst = 0.015, Fst = 0.018, P < 0.05) pools. These estimates of population genetic diversity and structure are critical measures for both species that will help inform recovery management actions.


Similar content being viewed by others
References
Alexander DG, Schlising RA (1998) Patterns in time and space for rare macroinvertebrates and vascular plants in vernal pool ecosystems at the Vina plains preserve, and implications for pool landscape management. In: Witham CW, Bauder ET, Belk D, Ferren WRJ, Ornduff R (eds) Ecology, conservation, and management of vernal pool ecosystems. Proceedings of the 1996 conference of the California Native Plant Society
Baskin Y (1994) California’s ephemeral vernal pools may be a good model for speciation. Bioscience 44:384–388
Beardsmore JA (1983) Extinction, survival and genetic variation. In: Schonewald-Cox CM, Chambers SM, MacBryde B, Thomas WL (eds) Genetics and conservation: a reference for managing wild animal and plant populations. Benjamin/Cummings, California, pp 125–151
Black C, Zedler PH (1998) An overview of 15 years of vernal pool restoration and construction activities in San Diego County, California. In: Witham CW, Bauder ET, Belk D, Ferren WRJ, Ornduff R (eds) Ecology, conservation, and management of vernal pool ecosystems. Proceedings of the 1996 conference of the California Native Plant Society
Boykin LM, Kubatko LS, Lowrey TK (2010) Comparison of methods for rooting phyologenetic trees: a case study using Orcuttieae (Poaceae: Chloridoideae). Mol Phylogenet Evol 54:687–700
CNDDB (2006) California Natural Diversity Database, California Department of Fish and Game. http://www.dfg.ca.gov/biogeodata/cnddb. Accessed July 2006
Cornuet JM, Piry S, Luikart G, Estoup A, Solignac M (1999) New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153:1989–2000
Davis HG, Sloop CM, Cushman JH (2009) Developing tools to promote the recovery of five federally listed vernal pool grasses. Final Report. United States Fish and Wildlife Service, Sacramento
Edmands S (2007) Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol Ecol 16:463–475
Elam D (1998) Population genetics of vernal pool plants: theory data and conservation implications. In: Witham CW, Bauder ET, Belk D, Ferren WRJ, Ornduff R (eds) Ecology, conservation, and management of vernal pool ecosystems. Proceedings of the 1996 conference of the California Native Plant Society
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Estoup A, Garnery L, Solignac M, Cornuet JM (1995) Microsatellite variation in honey bee (Apis mellifera L.) populations: hierarchical genetic structure and test of the infinite allele and stepwise mutation models. Genetics 140:679–695
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin ver 3.11: an integrated software package for population genetic data analysis. Evol Bioinform Online 1:47–50
Falush D, Stephans M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Fan Q, Chen S, Zhou R, Xiang X, Liao W, Shi S (2011) Genetic variation of wild litchi (Litchi chinensis Sonn. subsp. chinensis) revealed by microsatellites. Conserv Genet 12:753–760
Ferguson NJ, Ellstrand NC (1999) Assessment of seed bank buffering of genetic change in Dodeca hemaleptoceras (Slender-horned Spineflower). Final Report to M. Meyer. Department of Fish and Game, Sacramento
Ferren WRJ, Hubbard DM, Wiseman S, Parikh AK, Gale N (1998) Review of 10 years of vernal pool restoration and creation in Santa Barbara, California. In: Witham CW, Bauder ET, Belk D, Ferren WRJ, Ornduff R (eds) Ecology, conservation, and management of vernal pool ecosystems. Proceedings of the 1996 conference of the California Native Plant Society
Foin T, Reilly S, Pawley A, Ayres D, Carlson T, Hodem P, Switzer P (1998) Improving recovery planning for the conservation of threatened and endangered taxa. Bioscience 48:177–184
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Frankham R (2010) Where are we in conservation genetics and where do we need to go? Conserv Genet 11:661–663
Frankham R, Ballou JD, Brosco DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Gilligan DM, Briscoe DA, Frankham R (2005) Comparative losses of quantitative and molecular genetic variation in finite populations of Drosophila melanogaster. Genet Res 85:47–55
Gilpin ME, Soulé ME (1986) Minimum viable populations: processes of species extinction. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, pp 19–34
Gitzendanner MA, Soltis PE (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87(6):783–792
Griggs FT (1981) Life histories of vernal pool annual grasses. Fremontia 9:14–17
Griggs FT, Jain S (1983) Conservation of vernal pool plants in California, II. Population biology of a rare and unique grass genus Orcuttia. Biol Conserv 27:171–193
Hobson WA, Dahlgren RA (1998) A quantitative study of pedogenesis in California vernal pool wetlands. Soil Sci Soc Am Spec Publ 54:107–127
Holland R (1978) The geographic and edaphic distribution of vernal pools in the great central valley, California. California Native Plant Society Special Publication No. 4, Sacramento
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Karron JD (1987) A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evol Ecol 1:47–58
Keeler-Wolf T, Elam DR, Lewis K, Flint SA (1998) California vernal pool assessment preliminary report. State of California. Department of Fish and Game, Sacramento
Keeley JE (1998) C4 photosynthetic modifications in the evolutionary transition from land to water in aquatic grasses. Oecologia 116:85–97
Lewis PO, Zaykin D (2001) Genetic data analysis: computer program for the analysis of allelic data. Version 1.1 (d16c). Free program distributed by the authors over the internet from http://hydrodictyon.eeb.uconn.edu/people/plewis/software.php. Accessed 19 Jan 2011
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Martin MA, Mattioni C, Cherubini M, Taurchini D, Villani F (2010) Genetic diversity in European chestnut populations by means of genomic and genic microsatellite markers. Tree Genet Genomes 6:735–744
Miller MP (2005) Alleles in space: computer software for the joint analysis of inter individual spatial and genetic information. J Hered 96:722–724
Nikiforoff CC (1941) Hardpan and micro-relief in certain soil complexes of California. USDA Technical bulletin No. 745, Washington DC
Peakal R, Smouse PE (2006) GENAlEx 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A (2004) GeneClass2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure from multilocus genotype data. Genetics 155:945–959
Ramp Neal JM, Ranker TA, Collinge SK (2008) Conservation of rare species with island-like distributions: a case study of Lasthenia conjugens (Asteraceae) using population genetic structure and the distribution of rare markers. Plant Species Biol 23:97–110
Ramp JM, Collinge SK, Ranker TA (2006) Restoration genetics of the vernal pool endemic Lasthenia conjugens (Asteraceae). Conserv Genet 7:631–649
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237
Reeder JR (1982) Systematics of the tribe Orcuttieae (Gramineae) and the description of a new segregate genus Tuctoria. Am J Bot 69:1082–1095
Riley L, McGlaughlin ME, Helenurm K (2010) Genetic diversity following demographic recovery in the insular endemic plant Galium catalinense subspecies acrispum. Conserv Genet 11:2015–2025
Segelbacher G, Cushman SA, Epperson BK, Fortin MJ, Francois O, Hardy OJ, Holderegger R, Taberlet P, Waits LP, Manel S (2010) Applications of landscape genetics in conservation biology: concepts and challenges. Conserv Genet 11:375–385
Sloop, CM (in press) The population and genetic status of the endangered vernal pool annual Limnanthes floccosa Howell ssp. californica Arroyo: implications for species recovery. In: Schlising RA, DG Alexander (eds) Vernal pool conservation: research, progress and problems: is recovery possible? Studies from the Herbarium, California State University, Chico
Sloop, CM, Ayres DR (in press) Conservation genetics of two endangered vernal pool plants of the Santa Rosa Plain, Sonoma County. Proceedings of the CNPS conservation conference, 17–19 Jan 2009
Sloop CM, Eberl R, Ayres DR (In review) Genetic diversity and structure in the annual vernal pool endemic Limnanthes vinculans Ornduff (Limnanthaceae): implications of breeding system and restoration practices. Conserv Genet
Sloop CM, Pickens C, Gordon SP (2011) Conservation genetics of Butte County meadowfoam (Limnanthes floccosa ssp. californica Arroyo), an endangered vernal pool endemic. Conserv Genet 12:211–323
Solomeshch AI, Barbour MG, Holland RF (2007) Vernal pools. In: Barbour MG, Keeler-Wolf T, Schoenherr AA (eds) Terrestrial vegetation of California, 3rd edn. University of California Press, California, pp 394–424
Stone RD (1990) California’s endemic vernal pool plants: some factors influencing their rarity and endangerment. In: Ikeda DH, Schlising RA (eds) Vernal pools plants: their habitat and biology. Studies from the Herbarium, California State University, Chico
Sun G, Salomon B (2003) Microsatellite variability and heterozygote deficiency in the arctic-alpine Alaskan wheatgrass (Elymus alaskanus) complex. Genome 46:729–737
Tallmon DA, Luikart G, Waples RS (2004) The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol 19:489–496
US Fish and Wildlife Service (2005) Recovery plan for vernal pool ecosystems of California and Southern Oregon. http://www.fws.gov/sacramento/es/recovery_plans/vp_recovery_plan_links.htm. Accessed 11 Oct 2010
Weitkamp WA, Graham RC, Anderson MA, Amrhein C (1996) Pedogenesis of a vernal pool Entisol-Alfisol-Vertison catena in southern California. Soil Sci Soc Am 60:316–323
Young AG, Clarke GM (2000) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge
Acknowledgments
We thank the U.S. Fish & Wildlife Service (Agreement No.: 802706G122) for funding support. We thank Dr. Derek Girman for use of the Sonoma State Core DNA Facility and Dr. Richard Whitkus for providing laboratory and office space. We further acknowledge the many local experts, land managers, and landowners who helped locate and obtain access to a majority of the extant sites, including Joe Silveira (USFWS), Carol Witham (CNPS), John Vollmer and Rich Reiner (The Nature Conservancy).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gordon, S.P., Sloop, C.M., Davis, H.G. et al. Population genetic diversity and structure of two rare vernal pool grasses in central California. Conserv Genet 13, 117–130 (2012). https://doi.org/10.1007/s10592-011-0269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-011-0269-y