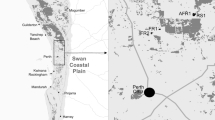
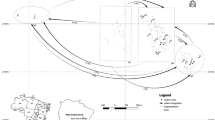
Abstract
Tropical trees are generally long-lived making it difficult to assess the long-term effects of habitat fragmentation on genetic diversity. Maintenance of genetic diversity in fragmented landscapes is largely dependent on the species’ mating system and the degree of genetic connectivity (seed and pollen flow) among fragments. Currently, these parameters are largely unknown for many endangered tropical tree species. Additionally, landscape fragmentation may isolate tropical tree populations from larger, more continuous populations. The role of isolated individuals in pollen transfer within and between remnant populations is not clear. In this study, we estimate the mating system and pollen flow patterns in continuous and remnant populations of the endangered tropical tree Guaiacum sanctum (Zygophyllaceae). Fractional paternity analyses were used to estimate average gene flow distances between fragmented remnant populations and the siring success of an intermediately located, but isolated individual. In these populations, G. sanctum is a mixed-mating species (t m = 0.72 − 0.95) whose pollen is transported over large distances (>4 km). An isolated tree may have functioned as a stepping-stone between two clusters of individuals, assisting long-distance pollen movement. This individual also sired a disproportionately high number of seeds (13.9%), and is thus an important component of the reproductive success of these populations, thus rejecting Janzen’s “living-dead” hypothesis. The high levels of genetic diversity maintained as a consequence of long-distance pollen-flow suggest that this endangered species may have the potential for future adaptation and population expansion if suitable habitats become available.





Similar content being viewed by others
References
Ahmed S, Compton SG, Butlin RK, Gilmartin PM (2009) Wind-borne insects mediate directional pollen transfer between desert fig trees 160 kilometers apart. Proc Natl Acad Sci 106:20342–20347
Aldrich PR, Hamrick JL (1998) Reproductive dominance of pasture trees in a fragmented tropical forest mosaic. Science 281:103–105
Apsit VJ, Hamrick JL, Nason JD (2001) Breeding population size of a fragmented population of a Costa Rican dry forest tree species. J Hered 92:415–420
Bawa KS, Bullock SH, Perry DR, Coville RE, Grayum MH (1985) Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. Am J Bot 72:346–356
Cascante A, Quesada M, Lobo JA, Fuchs EJ (2001) Effects of dry tropical forest fragmentation on the reproductive success and genetic structure of the tree Samanea saman. Conserv Biol 16:137–147
Chakraborty R, Meagher TR, Smouse PE (1988) Parentage analysis with genetic markers in natural populations. I. The expected proportion of offspring with unambiguous paternity. Genetics 118:527–536
Chase MR, Moller C, Kesseli R, Bawa KS (1996) Distant gene flow in tropical trees. Nature 383:398–399
CITES (2000) Proposal to transfer Guaiacum sanctum from Appendix II to Apendix I of CITES. Speller & Sons, New York
Devlin B, Roeder K, Ellstrand NC (1988) Fractional paternity assignment: theoretical development and comparison to other methods. Theor Appl Genet 76:369–380
Dick CW (2001) Genetic rescue of remnant tropical trees by an alien pollinator. Proc R Soc Lond 268:2391–2396
Dick CW, Etchelecu G, Austerlitz F (2003) Pollen dispersal of tropical trees (Dinizia excelsa: Fabaceae) by native insects and African honeybees in pristine and fragmented Amazonian rainforest. Mol Ecol 12:753–764
Dick CW, Hardy OJ, Jones FA, Petit RJ (2008) Spatial scales of pollen and seed-mediated gene flow in tropical rain forest trees. Trop Plant Biol 1:20–33
Ehrlich PR, Raven PH (1969) Differentiation of populations. Science 165:1228–1232
Frankie G, Haber W (1983) Why bees move among mass-flowering neotropical trees. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Neinhold, New York, USA, pp 360–372
Frankie GW, Opler PA, Bawa KS (1976) Foraging behaviour of solitary bees: implications for outcrossing of a neotropical forest tree species. J Ecol 64:1049–1057
Fuchs EJ, Hamrick JL (2010a) Genetic diversity in the endangered tropical tree, Guaiacum sanctum (Zygophyllaceae). J Hered 101:284–291
Fuchs EJ, Hamrick JL (2010b) Spatial genetic structure within size classes of the endangered tropical tree Guaiacum sanctum (Zygophyllaceae). Am J Bot 97:1200–1207
Fuchs EJ, Lobo J, Quesada M (2003) Effects of forest fragmentation and flowering phenology on the reproductive success and mating patterns of the tropical dry forest tree Pachira quinata. Conserv Biol 17:149–157
Geist HJ, Lambin EF (2004) Dynamic causal patterns of desertification. BioSci 54:817–829
Gerber S, Chabrier P, Kremer A (2003) FAMOZ: a software for parentage analysis using dominant, codominant and uniparentally inherited markers. Mol Ecol Notes 3:479–481
González-Rivas B, Tigabu M, Gerhardt K, Castro-Marín G, Odén P (2006) Species composition, diversity and local uses of tropical dry deciduous and gallery forests in Nicaragua. Biodivers Conserv 15:1509–1527
Hamilton MB (1999) Tropical tree gene flow and seed dispersal. Nature 401:129–130
Hamrick JL (1994) Genetic diversity and conservation in tropical forests. In: Drysdale RM, John S, Yapa A (eds) Proceedings international symposium on genetic conservation and production of tropical forest tree seed. Asian-Canada Forest Tree Seed Centre, Muak-Lek, Saraburi, Thailand, pp 1–9
Hamrick JL, Apsit VJ (2004) Breeding structure of neotropical dry forest tree species in fragmented landscapes. In: Frankie GW, Mata A, Vinson SB (eds) Biodiversity conservation in Costa Rica. Learning the lessons in a seasonal dry forest. University of California Press, Berkley, CA, USA, pp 30–37
Hamrick JL, Nason JD (1996) Consequences of dispersal in plants. In: Rhodes OE, Chesser RK, Smith MH (eds) Population dynamics in ecological space and time. University of Chicago Press, Chicago, pp 203–236
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. New For 6:95–124
Holdrige LR, Poveda LJ (1975) Árboles de Costa Rica. Centro Científico Tropical, San José, Costa Rica
Janzen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171:203–205
Janzen DH (1986) Blurry catastrophes. Oikos 47:1–2
Janzen DH (1988) Tropical dry forests: the most endangered major tropical ecosystem. In: Wilson EO (ed) Biodiversity. National Academy Press, Washington, DC, pp 130–137
Jiménez QM (1993) Árboles maderables en peligro de extinción en Costa Rica. Museo Nacional de Costa Rica, San Jose, Costa Rica
Kenta T, Isagi Y, Nakagawa M, Yamashita M, Nakashizuka T (2004) Variation in pollen dispersal between years with different pollination conditions in a tropical emergent tree. Mol Ecol 13:3575–3584
Konuma A, Tsumura Y, Lee CT, Lee SL, Okuda T (2000) Estimation of gene flow in the tropical-rainforest tree Neobalanocarpus heimii (Dipterocarpaceae), inferred from paternity analysis. Mol Ecol 9:1843–1852
Latouche-Hallé C, Ramboer A, Bandou E, Caron H, Kremer A (2004) Long-distance pollen flow and tolerance to selfing in a neotropical tree species. Mol Ecol 13:1055–1064
Levin DA, Kerster HW (1970) Phenotypic dimorphism and population fitness in Phlox. Evolution 24:128–134
Levin D, Kerster H (1974) Gene flow in seed plants. Evol Biol 7:139–220
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst 15:65–95
Loveless MD, Hamrick JL, Foster RB (1998) Population structure and mating system in Tachigali versicolor, a monocarpic neotropical tree. Heredity 81:134–143
Lowe AJ, Boshier D, Ward M, Bacles CFE, Navarro C (2005) Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for neotropical trees. Heredity 95:255–273
Manchenko GP (1994) Handbook of detection of enzymes on electrophoretic gels. CRC Press, Ann Arbor, MI
Meagher TR, Thompson E (1986) The relationship between single parent and parent pair genetic likelihoods in genealogy reconstruction. Theor Pop Biol 29:87–106
Murawski DA, Hamrick JL (1991) The effect of the density of flowering individuals on the mating systems of nine tropical tree species. Heredity 67:167–174
Murawski DA, Hamrick JL (1992) Mating system and phenology of Ceiba pentandra (Bombacaceae) in central Panama. J Hered 83:401–404
Nason JD, Hamrick JL (1997) Reproductive and genetic consequences of forest fragmentation: two case studies of neotropical canopy trees. J Hered 88:264–276
Nason JD, Herre EA, Hamrick JL (1996) Paternity analysis of the breeding structure of strangler fig populations: evidence for substantial long-distance wasp dispersal. J Biogeogr 23:501–512
Nason JD, Herre EA, Hamrick JL (1998) The breeding structure of a tropical keystone plant resource. Nature 391:685–687
Oldfield S, Lusty C, MacKinven A (1998) The world list of threatened trees. World Conservation Press, Cambridge
Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG (2005) Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol 14:689–701
Quesada M, Fuchs EJ, Lobo JA (2001) Pollen load size, reproductive success and progeny kinship of natural pollinated flowers of the tropical dry forest tree, Pachira quinata (Bombacaceae). Am J Bot 88:2113–2118
Quesada M, Stoner KE, Rosas-Guerrero V, Palacios-Guevara C, Lobo JA (2003) Effects of habitat disruption on the activity of nectarivorous bats (Chiroptera: Phyllostomidae) in a dry tropical forest: implications for the reproductive success of the neotropical tree Ceiba grandiflora. Oecologia 135:400–406
Ritland K (2002) Extensions of models for the estimation of mating systems using n independent loci. Heredity 88:221–228
Roubik DW (1992) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge, Massachusetts, USA
Schnabel A, Hamrick JL (1995) Understanding the population genetic structure of Gleditsia triacanthos l. The scale and pattern of pollen gene flow. Evolution 49:921–931
Smouse PE, Sork VL (2004) Measuring pollen flow in forest trees: an exposition of alternative approaches. For Ecol Manag 197:21–38
Soltis DE, Haufler CH, Darrow DC, Gastony GJ (1983) Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. Am Fern J 73:9–27
Sork V, Smouse P (2006) Genetic analysis of landscape connectivity in tree populations. Landsc Ecol 21:821–836
Streiff R, Ducousso A, Lexer C, Steinkellner H, Gloessl J, Kremer A (1999) Pollen dispersal inferred from paternity analysis in a mixed oak stand of Quercus robur L. and Q. petrae Liebl. Mol Ecol 8:831–841
Trapnell DW, Hamrick JL (2004) Partitioning nuclear and chloroplast variation at multiple spatial scales in the neotropical epiphytic orchid, Laelia rubescens. Mol Ecol 13:2655–2666
Voeks RA (2004) Disturbance pharmacopoeias: medicine and myth from the humid tropics. Ann Assoc Am Geogr 94:868–888
Wendel JF, Weeden NF (1989) Visualization and interpretation of plant isozymes. In: Soltis D, Soltis P (eds) Isozymes in plant biology. Advances in plant sciences series. Dioscorides Press, Portland, OR, pp 5–45
White GM, Boshier DH, Powell W (2002) Increased pollen flow counteracts fragmentation in a tropical dry forest: an example from Swietenia humilis Zuccarini. Proc Natl Acad Sci 99:2038–2042
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Acknowledgments
The authors would like to thank T. Robles; R. Blanco and M. Chavarria from Área de Conservación Guanacaste (ACG); C. Alvarado and U. Chavarría from ACAT; C. Deen, and D. Trapnell for technical assistance. J Ross-Ibarra and M Poelchau and two anonymous reviewers provided valuable comments on earlier versions of this manuscript. This work was supported by Idea Wild; Universidad de Costa Rica (Vicerrectoría de Investigación) and Organization for Tropical Studies [to E.J.F.] and National Science Foundation [0211526 to J.L.H.].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchs, E.J., Hamrick, J.L. Mating system and pollen flow between remnant populations of the endangered tropical tree, Guaiacum sanctum (Zygophyllaceae). Conserv Genet 12, 175–185 (2011). https://doi.org/10.1007/s10592-010-0130-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0130-8