Abstract
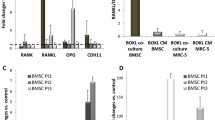
After prostate cancer cells (PCa) arrive in bone, interactions with cells that include long bone osteoblasts (LBOB) and bone marrow stromal cells (BMSC) lead to metastasis formation. The effect of heterotypic cell–cell contact between PCa cells and BMSC or LBOB on PCa cell gene expression is poorly understood. To establish the role of heterotypic contact in bone metastasis formation, we mixed and co-cultured PC3 cells with rat BMSC, LBOB, or human prostate stromal cells (PS15). PC3 cells were then re-isolated for gene array analysis, and imaged using in situ hybridization to confirm that heterotypic contact regulates gene expression. The gene expression was examined using focused gene arrays containing 96 each of tumor metastasis genes or osteogenesis genes. A total of 18 out of 192 genes in PC3 cells were found to be under or over expressed subsequent to heterotypic contact with BMSC when analyzed. A total of 15 genes out of 192 were regulated in co-culture with LBOB, and 19 genes with PS15. Only two genes, uPA and Collagen III, were regulated by contact with BMSC or LBOB (both are bone derived cells), but not by contact with PS15. The relationship between cell–cell contact and uPA expression was further explored by varying cell ratios in co-culture. uPA over-expression in PC3 was related to the BMSC:PC3 ratio, and was maximum at a 10:1 ratio, where most PC3 cells would be in contact with BMSC, as predicted by a theoretical model of heterotypic contact. In situ staining of micropatterned PC3 and BMSC cells showed that uPA over-expression localizes to regions of heterotypic cell–cell contact. Taken together, our results suggest that heterotypic cell-to-cell contact between PC3 and BMSC proportionally enhances gene expression for uPA, providing a mechanism for localized control of invasiveness.





Similar content being viewed by others
Abbreviations
- PCa:
-
Prostate cancer cell
- LBOB:
-
Long bone osteoblast
- BMSC:
-
Bone marrow stromal cell
- FACS:
-
Fluorescence-activated cell sorting
- TMG:
-
Tumor metastasis gene
- OG:
-
Osteogenesis gene
- PDMS:
-
Polydimethylsiloxane
- uPA:
-
Urokinase plasminogen activator
- ISH:
-
In situ hybridization
- ECM:
-
Extracellular matrix
References
Zhang X, Jin TG, Yang H et al (2004) Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res 64(19):7086–7091
Shah RB, Mehra R, Chinnaiyan AM et al (2004) Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 64(24):9209–9216
Kim SJ, Uehara H, Karashima T et al (2003) Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res 9(3):1200–1210
Kim SJ, Uehara H, Yazici S et al (2004) Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res 64(12):4201–4208
Patel P, Ashdown D, James N (2004) Is gene therapy the answer for prostate cancer? Prostate Cancer Prostatic Dis 7(Suppl 1):S14–S19
Harms JF, Welch DR (2003) MDA-MB-435 human breast carcinoma metastasis to bone. Clin Exp Metastasis 20(4):327–334
Rosol TJ, Tannehill-Gregg SH, LeRoy BE et al (2003) Animal models of bone metastasis. Cancer 97(3 Suppl):748–757
Festuccia C, Angelucci A, Gravina GL et al (2000) Osteoblast-derived TGF-beta1 modulates matrix degrading protease expression and activity in prostate cancer cells. Int J Cancer 85(3):407–415
Hart CA, Scott LJ, Bagley S et al (2002) Role of proteolytic enzymes in human prostate bone metastasis formation: in vivo and in vitro studies. Br J Cancer 86(7):1136–1142
Lang SH, Clarke NW, George NJ et al (1998) Interaction of prostate epithelial cells from benign and malignant tumor tissue with bone-marrow stroma. Prostate 34(3):203–213
Millimaggi D, Festuccia C, Angelucci A et al (2006) Osteoblast-conditioned media stimulate membrane vesicle shedding in prostate cancer cells. Int J Oncol 28(4):909–914
Wang J, Levenson AS, Satcher RL Jr (2006) Identification of a unique set of genes altered during cell–cell contact in an in vitro model of prostate cancer bone metastasis. Int J Mol Med 17(5):849–856
Nauman EA, Satcher RL, Keaveny TM et al (2001) Osteoblasts respond to pulsatile fluid flow with short-term increases in PGE(2) but no change in mineralization. J Appl Physiol 90(5):1849–1854
Hung CT, Pollack SR, Reilly TM et al (1995) Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop Relat Res (313):256–269
Kassen A, Sutkowski DM, Ahn H et al (1996) Stromal cells of the human prostate: initial isolation and characterization. Prostate 28(2):89–97
Sensibar JA, Pruden SJ, Kasjanski RZ et al (1999) Differential growth rates in stromal cultures of human prostate derived from patients of varying ages. Prostate 38(2):110–117
Satcher RL Jr, Dvorkin K, Levenson AS et al (2004) Gene expression in cancer cells is influenced by contact with bone cells in a novel coculture system that models bone metastasis. Clin Orthop Relat Res (426):54–63
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8(2):98–101
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2(8):584–593
Sung SY, Chung LW (2002) Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation 70(9–10):506–521
Hart CA, Brown M, Bagley S et al (2005) Invasive characteristics of human prostatic epithelial cells: understanding the metastatic process. Br J Cancer 92(3):503–512
Andreasen PA, Kjoller L, Christensen L et al (1997) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72(1):1–22
Festuccia C, Dolo V, Guerra F et al (1998) Plasminogen activator system modulates invasive capacity and proliferation in prostatic tumor cells. Clin Exp Metastasis 16(6):513–528
Margheri F, D’Alessio S, Serrati S et al (2005) Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther 12(8):702–714
Pulukuri SM, Gondi CS, Lakka SS et al (2005) RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem 280(43):36529–36540
Angelucci A, Gravina GL, Rucci N et al (2006) Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer 13(1):197–210
Benasciutti E, Pages G, Kenzior O et al (2004) MAPK and JNK transduction pathways can phosphorylate Sp1 to activate the uPA minimal promoter element and endogenous gene transcription. Blood 104(1):256–262
Bhatia SN, Balis UJ, Yarmush ML et al (1999) Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. Faseb J 13(14):1883–1900
Chung LW, Baseman A, Assikis V et al (2005) Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 173(1):10–20
Siggelkow H, Rebenstorff K, Kurre W et al (1999) Development of the osteoblast phenotype in primary human osteoblasts in culture: comparison with rat calvarial cells in osteoblast differentiation. J Cell Biochem 75(1):22–35
Festuccia C, Guerra F, D’Ascenzo S et al (1998) In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int J Cancer 75(3):418–431
Acknowledgments
The authors would like to acknowledge Dr. Chung Lee for his generous support. The work in this manuscript was supported by a research grant from the Robert Wood Johnson Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Wang, J., Bilen, M.A. et al. Modulation of prostate cancer cell gene expression by cell-to-cell contact with bone marrow stromal cells or osteoblasts. Clin Exp Metastasis 26, 993–1004 (2009). https://doi.org/10.1007/s10585-009-9289-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-009-9289-0