Abstract
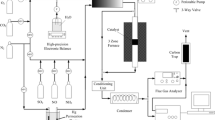
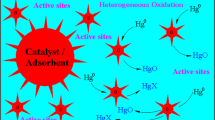
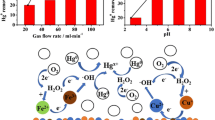
The heterogeneous mercury reaction mechanism, reactions among elemental mercury (Hg0) and simulated flue gas across laboratory-scale selective catalytic reduction (SCR) reactor system was studied. The surface of SCR catalysts used in this study was analyzed to verify the proposed reaction pathways using transmission electron microscopy with energy dispersive X-ray analyses (TEM-EDX) and X-ray photoelectron spectroscopy (XPS). The Langmuir–Hinshelwood mechanism was proven to be most suitable explaining first-layer reaction of Hg0 and HCl on the SCR catalyst. Once the first layer is formed, successive layers of oxidized mercury (HgCl2) are formed, making a multi-layer structure.







Similar content being viewed by others
References
Galbreath K, Zygarlicke C (1996) Environ Sci Technol 30:2421
Serre S, Silcox G (2000) Ind Eng Chem Res 39:1723
Pavlish J, Sondreal E, Mann M, Olson E, Galbreath K, Laudal E, Benson S (2003) Fuel Proc Technol 82:89
Galbreath KC, Zygarlicke CJ (2000) Fuel Process Technol 65–66:289
Lee TG, Hedrick E, Biswas P (2002) J Air Waste Manage Assoc 52:1316
Senior CL, Bool LE, Huffman GP, Huggins FE, Shah N, Sarofim A, Olmez I, Zeng T (1997) Proceedings of the 90th Air & Waste Management Association annual meeting. Toronto, Canada
Lietti L, Ramis G, Berti F, Toledo G, Robba D, Busca G, Forzatti D (1998) Catal Today 42:101
Niksa S, Fujiware N (2005) J Air Waste Manage Assoc 55:1866
Richardson C, Machalek T, Miller S, Dene C, Chang R (2002) J Air Waste Manage Assoc 52:941
Srivastava R, Hutson N, Martin B, Princiotta F, Staudt J (2006) Environ Sci Technol 40:1385
Laudal DL, Thompson JS, Pavlish JH, Brickett L, Chu P, Srivastava RK, Lee CW, Kilgroe J (2003) Environ Manage 1:16
Glenn AN, Hongqun Y, Robert CB, Dennis LL, Grant ED, John E (2003) Fuel 82:107
Carl R, Tom M, Scott M, Chuck D, Ramsay C (2002) J Air Waste Manage Assoc 52:941
ASTM D6784 (2002) Annual book of ASTM standards, vol 11.03. ASTM International, PA
Jossena R, Heinea MC, Pratsinisa SE, Augustineb SM, Akhtarb MK (2007) Appl Catal B 69:181
Briggs D, Seah MP (1996) Practical surface analysis (Appendix 5). John Wiley & Sons, Inc., New York
Hutson ND, Attwood B, Schecke K (2007) Environ Sci Technol 41:1747
Dupin J, Gobeau D, Vinatier P, Levasseur A (2000) Phys Chem Chem Phys 2:1319
Galbreath KC, Zygarlicke CJ (1996) Environ Sci Technol 30:2421
Hal B, Schager P, Lindqvist O (1991) Water Air Soil Pollut 56:3
Eswaran S, Stenger H (2005) Energy Fuels 19:2328
Senior CL (2006) J Air Waste Manage Assoc 56:23
Pilling M, Seakins PW (1995) Reaction kinetics. Oxford Science Publications, New York
Niksa S, Fujiwara N (2005) J Air Waste Manage Assoc 55:930
Acknowledgements
This work was supported by grant No. (R01-2006-000-10007-0) from the Basic Research Program of the Korea Science & Engineering Foundation and by Ministry of Environment as the Eco-technopia 21 project (No. 2007-01003-0059-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eom, Y., Jeon, S.H., Ngo, T.A. et al. Heterogeneous Mercury Reaction on a Selective Catalytic Reduction (SCR) Catalyst. Catal Lett 121, 219–225 (2008). https://doi.org/10.1007/s10562-007-9317-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9317-0