Abstract
Purpose
The objective was to systematically review clinical trial data on the effects of statins on high-density lipoproteins (HDL) and to examine the possibility that this provides cardiovascular benefits in addition to those derived from reductions in low-density lipoproteins (LDL).
Methods
The PubMed database was searched for publications describing clinical trials of atorvastatin, pravastatin, rosuvastatin, and simvastatin. On the basis of predefined criteria, 103 were selected for review.
Results
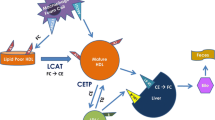
Compared with placebo, statins raise HDL, measured as HDL-cholesterol (HDL-C) and apolipoprotein A-I (apo A-I); these elevations are maintained in the long-term. In hypercholesterolemia, HDL-C is raised by approximately 4% to 10%. The percentage changes are greater in patients with low baseline levels, including those with the common combination of high triglycerides (TG) and low HDL-C. These effects do not appear to be dose-related although there is evidence that, with the exception of atorvastatin, the changes in HDL-C are proportional to reductions in apo B-containing lipoproteins. The most likely explanation is a reduced rate of cholesteryl ester transfer protein (CETP)-mediated flow of cholesterol from HDL. There is some evidence that the statin effects on HDL reduce progression of atherosclerosis and risk of cardiovascular disease independently of reductions in LDL.
Conclusion
Statins cause modest increases in HDL-C and apo A-I probably mediated by reductions in CETP activity. It is plausible that such changes independently contribute to the cardiovascular benefits of the statin class but more studies are needed to further explore this possibility.
Similar content being viewed by others
1 Introduction
It has been known for over 30 years that circulating levels of high-density lipoprotein (HDL) are inversely associated with the risk of atherosclerotic diseases [1]. Population studies, in which HDL was measured as HDL-cholesterol (HDL-C) or apolipoprotein A-I (apo A-I), have repeatedly confirmed the strong and apparently independent relationship with risk of coronary heart disease (CHD) [2–4]. A partial understanding of HDL metabolism has emerged in this period, particularly regarding the role of apo A-I, the major protein component of HDL, in promoting cholesterol efflux from foam cell macrophages of atherosclerotic lesions [5, 6]. According to current models, HDL operates as a dynamic system, removing excess cholesterol from cells, transporting it to the liver for excretion, and ensuring overall cholesterol homeostasis in the body. Recent laboratory evidence suggests that HDL may in addition retard atherosclerosis through antioxidant and anti-inflammatory actions [7, 8]. Evidence from both experimental and population studies has led to the concept of HDL as a protective system, mitigating the damaging effects on the vessel wall of apo B-containing low and very-low-density lipoproteins (LDL and VLDL). HDL is metabolically interrelated with the apo B-containing lipoproteins; low HDL-C levels are commonly found in subjects with elevated serum triglycerides (TG) and VLDL, a profile that carries a high risk of CHD [2]. Moreover, low HDL-C levels are frequent in those with obesity, metabolic syndrome or type 2 diabetes (T2D), conditions in which TG metabolism is abnormal [9]. HDL levels are also affected by some lifestyle factors such as exercise and smoking. In addition, there are a number of important independent genetic determinants [10].
The guidelines issued by the Adult Treatment Panel III of the National Cholesterol Education Programme [11] identify low HDL-C level as an important risk factor for cardiovascular disease (CVD) and as a criterion for the initiation of lifestyle changes, and use of therapeutic agents. Nevertheless, treatment emphasis remains firmly on reduction of LDL-C, primarily using HMG-CoA reductase inhibitors (statins), which very effectively reduce circulating levels of atherogenic LDL via upregulation of LDL receptors. The clinical success of statins confirms the epidemiological evidence and points to elevated LDL as a direct cause of atherosclerosis progression [12]. However, statins also increase HDL, measured as HDL-C or apo A-I, and, based on the strong epidemiologic evidence, it is possible that this action may independently contribute to their benefits.
From the combined data from four large prospective studies, Gordon et al. [13] estimated that an increment of 1 mg/dl in HDL-C is associated with a 2–3% lower risk of CHD. Thus, a relatively modest statin-mediated effect on HDL-C, for example, an increase of 10% that is possible with statins [14], might provide important protection from CVD in addition to the undoubted benefits of LDL reduction. However, extrapolation from epidemiology to putative benefits of drug therapy requires caution. Support for the concept of HDL-raising by drugs comes from prospective trials of fibrates and of nicotinic acid, two drug types with substantial effects on HDL and VLDL metabolism [15–18], and also from early clinical work with infusions of apo A-I in liposomes where dramatic reductions in coronary atheroma have been observed [19]. In contrast, recent trials of torcetrapib, an inhibitor of cholesteryl ester transfer protein (CETP) that induces substantial increases in HDL-C and apo A-I failed to show any benefit on atherosclerosis [20–22]. It is possible that the plasma concentrations of HDL-C or apo A-I may not always reflect the protective activity of the HDL system. It is important to consider both the magnitude of effect on HDL level and the mechanism by which it is achieved. Two explanations have been proposed for the effects of statins on HDL. In cell experiments, inhibition of HMG-CoA reductase by statins was shown to increase peroxisome-proliferator receptor activator-alpha (PPARα) activity and, like fibrates, to elevate the hepatic synthesis of apo A-I [23]. Such an effect would be expected to increase the formation of HDL precursor particles. A second explanation revolves around the metabolic relationship between HDL and TG-rich atherogenic lipoproteins. Guerin et al. [24] showed that atorvastatin reduced circulating levels of CETP, and also importantly the rate of CETP-mediated CE transfer from HDL to VLDL secondary to reduction in the latter. Statins, however, are not known to be direct inhibitors of CETP.
With statins, elevations in HDL-C range between 3% and 15%. These are relatively modest increases compared with agents such as nicotinic acid, fibrates, and the recently developed CETP inhibitors. However, given the widespread use of statins and the putative benefit of HDL elevation, it is important to thoroughly assess this aspect of statin action. In this review we consider: (1) data from placebo-controlled trials to confirm that statins have real effects on HDL, (2) how effects on HDL compare between statins when administered at recommended starting doses, (3) dose-related effects of statins on HDL and comparative data at the highest prescribed doses, (4) the effects of statins in patients with mixed hyperlipidemia and diabetes, and (5) the longer-term effect of statins on HDL and evidence linking this to clinical benefit.
2 Methods
For this review, searches were conducted for publications that appeared on the PubMed database up to the end of 2007 that describe clinical trials of atorvastatin, pravastatin, rosuvastatin, and simvastatin; this generated 2,630 citations. The studies varied widely with respect to populations studied, treatment duration, and design. Selection from the total was based on predefined criteria. Only articles that provided HDL data from randomized clinical trials were included (almost 50% of the total). Sub studies were not included unless they were particularly pertinent. Since variability in change in HDL parameters with statin treatment greatly exceeds that for LDL, only studies that utilized ≥50 patients per group in parallel design or ≥30 in a crossover design were included. In addition, only those studies with a dietary lead-in period ≥2 weeks (given the influence of diet on HDL levels) and a treatment period ≥6 weeks were included; 47% of studies were excluded on these design criteria.
Finally, we considered the method by which HDL-C had been measured. The most commonly used assays are those in which it is measured after apo B-containing lipoproteins are precipitated from plasma with polyvalent anion and divalent metal cation; one such is the reference method of the US Center for Disease Control. Recently developed homogeneous assays employ a variety of reagents to block apo B-containing lipoproteins, allowing assay only of HDL-C. However, questions remain as to the specificity of these assays under contrasting conditions of high and low LDL levels as typically observed in statin trials [25]. Only those studies were included in which HDL-C was confirmed in the publication or after enquiry to the lead author as having been measured using a precipitation method. Fifteen percent of the remaining citations were excluded on this basis leaving 103 publications that conformed to our criteria; these form the basis of our review.
3 Effects of statins on HDL: evidence from placebo-controlled trials
Most clinical trials of statins were designed with LDL-cholesterol (LDL-C) as the primary endpoint and conducted in patients with hypercholesterolemia, excluding those with very high TG. Usually the study populations had normal HDL-C levels on average and were treated for at least 6 weeks. In the placebo-controlled trial of Hunninghake et al. [26], a study was made of the rate of change of lipoprotein parameters in response to a range of doses of pravastatin. Patients were randomized following dietary stabilization and then treated for 12 weeks. In the pravastatin groups, HDL-C increased from baseline, reached maximal levels at 4 weeks and remained steady thereafter, whilst in the placebo group it was almost unchanged. At week 12, pravastatin had increased HDL-C by 6–7% from baseline, compared with 1% for placebo. The observed time course of the HDL-C increases with pravastatin paralleled the reductions in both LDL-C and TG, suggesting a mechanistic relationship between the changes. A treatment period of ≥6 weeks (one of our selection criteria) appears adequate for assessing the effects of statins on HDL-C.
Statin-induced effects on HDL are relatively small compared with those of LDL and, as a result, most clinical trials of statins are underpowered with respect to HDL parameters. In this review, the emphasis is initially placed therefore on those studies with the largest sample sizes. To allow comparison between trials, data relating to errors of the estimates for changes in HDL-C and apo A-I have been converted to 95% confidence intervals. Figure 1 describes the findings from the four largest placebo-controlled statin trials, which measured both HDL-C and apo A-I and reported the errors of the estimates of change. All four trials were in patients with primary hypercholesterolemia with TG levels <450 mg/dl. Patients were randomized in a parallel design following a dietary lead period ≥2 weeks. In two of the studies [29, 30], the errors of estimates are reported only for the pooled 10–80 mg simvastatin dose groups. These pooled data are shown in Fig. 1 to allow comparison with placebo. It is apparent that the percentage changes in HDL-C in the statin-treated groups are consistently greater than placebo; the lack of overlap in the 95% confidence limits suggests real drug responses. A similarly large study of pravastatin 20 mg [31] found a 7% increase in HDL-C compared with placebo (p < 0.001). Over the four trials the magnitude of effect on apo A-I was consistently less than on HDL-C, and not statistically different from placebo in two of them. This suggests that statins change HDL to a more cholesterol-rich form.
Eight other smaller placebo-controlled trials (n ≤ 106) in hypercholesterolemic patients of duration ≤6 months also fitted our selection criteria [32–39]. In 14 of the 15 statin dose groups comprising these studies, the mean change in HDL-C was numerically greater than in the corresponding placebo group. This was also the case for apo A-I in all nine groups in which it was measured. However, differences vs. placebo were not always significant, perhaps reflecting smaller group sizes. Similar findings were reported in a 12-week study in patients of African-American descent, where the HDL-C response to pravastatin was numerically greater but not significantly different from placebo [40].
It is important to consider whether statin-induced changes in HDL, like those in LDL, can be maintained over the long term. Keech et al. [41] measured the effects of simvastatin 20 and 40 mg over 3 years in patients considered to be at risk of CHD. Although this study did not include a dietary lead-in period, changes from baseline in the placebo group were minor. In the combined simvastatin groups, significant increases in HDL-C of 8–10% vs. placebo were maintained over 3 years while increases in apo A-I were also significant vs. placebo (5% measured up to 2 years only). HDL-C and occasionally also apo A-I have been monitored in large-scale placebo-controlled studies employing atherosclerosis progression or cardiovascular event endpoints, conducted for ≥2 years. The HDL-C data from these trials are summarized in Table 1. In general, the study populations were more inclusive than in shorter-term studies. In all but one trial, there was a positive effect on HDL-C (range, 1.5–10%) relative to placebo. The apo A-I responses when measured were lower and relatively variable. Data from these longer-term trials are consistent with those of shorter lipid-endpoint studies bearing in mind that compliance often decreases as the duration of the study increases.
The relatively large sample sizes in outcomes studies provide opportunities to identify factors contributing to the HDL response and thereby deduction as to possible mechanisms. In the untreated population, there is a well-established inverse curvilinear relationship between plasma levels of TG and HDL-C, with most variation in HDL-C being apparent at TG levels <220 mg/dl [56]. This reflects in part the action of CETP since high VLDL-TG levels facilitate the two-way transfer of TG and cholesteryl ester between VLDL and HDL. In a follow-up to the West of Scotland Coronary Prevention Study (WOSCOPS) [47], Streja et al. [57] analyzed data from those participants in the active group who were fully compliant (about half). Mean percent change in the pravastatin group decreased across quintiles of baseline HDL-C, such that the absolute increment remained relatively constant. Independent contributors to statin-induced change in HDL-C were alcohol intake, body mass index, and reduction in plasma TG, all of which have influence on plasma CETP levels and/or activity. Most likely the reduction in cholesteryl ester transfer from HDL that results in elevation of HDL-C is governed by the degree of reduction in VLDL [24]. The effect of statin would be to shift the patient’s position on the population curve relating HDL-C to TG levels. Such a mechanism involving CETP would be consistent with alteration of the particle size distribution of HDL towards larger, relatively cholesterol-rich particles that are characteristic of healthy low-risk populations manifesting the ideal profile of low TG and high HDL-C. Such statin-induced changes in subpopulations of HDL have been consistently demonstrated in the studies of Asztalos et al. [58–61].
Hepatic lipase is another factor with a key role in TG and lipoprotein metabolism. Increased hepatic lipase due to a common polymorphism (−514 C→T) is associated with lower levels of the larger HDL particles (HDL2) as well as greater numbers of small dense (more atherogenic LDL) [62–66]. In an intervention trial with combination lipid-lowering therapy including lovastatin [65], subjects homozygous for this allele had the highest hepatic lipase activity, lowest HDL2-C and the most angiographic improvement compared to those heterozygous or lacking the allele.
In summary, data from placebo-controlled trials in hypercholesterolemic subjects confirm that statins cause definite increases in HDL-C; the mechanism most likely involves reduced transfer of cholesteryl ester from HDL to VLDL but other factors such as hepatic lipase and other statin-induced effects may also contribute. The data also suggest that statin effects on HDL-C and apo A-I are maintained over time and that a treatment period of 6 weeks is sufficient to assess and compare different statins in this respect.
4 Comparative effects of statins on HDL at recommended starting doses
In clinical practice, most patients remain on the statin dose first prescribed [67] so it is appropriate to first compare the effects on HDL of statins when used at their recommended starting doses: atorvastatin 10 or 20 mg, pravastatin 20 or 40 mg, rosuvastatin 5 or 10 mg, and simvastatin 20 or 40 mg. Figure 2 shows data from the four largest comparative trials (n ≥ 156 per group) conducted to date that included these starting doses and met our criteria. Blasetto et al. [71] reported data from five individual trials prospectively designed for pooling into two sets (1) [29, 74, 75] and (2) [76, 77]. All trials were of parallel design with treatment duration ≥6 weeks, measured both HDL-C and apo A-I and reported the errors of the estimates of percentage change. Eligible patients included those with hypercholesterolemia, a relatively normal HDL-C level, TG <400 mg/dl, with or without high risk, manifest CHD or T2D.
Effects of statins given at recommended start doses on a high-density lipoprotein cholesterol (HDL-C) and b apolipoprotein A-I (apo A-I) in hypercholesterolemic patients. Data are shown as mean percent change from baseline ±95% confidence limits when available. Number at the base of the columns refers to the study as indicated in the table
Figure 2a shows there were no consistent differences in HDL responses between the upper and lower starting doses of each statin. Between the statins there is a tendency for rosuvastatin to have the greatest effect on both HDL-C and apo A-I. Mean percent changes in HDL-C from baseline across the trials weighted by patient numbers were as follows: rosuvastatin 8.5%, pravastatin 6.5%, simvastatin 6.4%, atorvastatin 5.5%. In some but not all trials, significant differences have been reported in the HDL-C response to the starting doses of rosuvastatin and atorvastatin. For example, in the largest study [68], the effect of rosuvastatin 10 mg on HDL-C (9.2%) was significantly greater than either atorvastatin 10 mg (6.8%, p < 0.01) or 20 mg (5.7%, p < 0.0001). Apo A-I responses show similar trends between statins but are generally lower than for HDL-C, with greater variability (Fig. 2b). Two further large trials compared atorvastatin and simvastatin [78, 79, data not shown]. Olsson et al. [78] (n ≥ 535 per group) reported notably lower HDL-C responses than in the trials described above although the effect of simvastatin on HDL-C (3.3%) was significantly greater than for atorvastatin (−0.1%). The same was true of apo A-I (0.8% vs. −1.8%). However, no difference was apparent between these statins in the study of Barter and O’Brien [79]. Moreover, Insull et al. [80] compared atorvastatin 10 mg, rosuvastatin 10 mg, and simvastatin 20 mg and found that all increased HDL-C to similar extents (6–7%).
In addition to those described above, 31 further trials in hypercholesterolemic patients that included starting doses of one or more statins met our selection criteria. In 17 of these [74, 79, 81–95], the overall changes in HDL-C were consistent with those shown in Fig. 2a in that they fell within the ranges of confidence limits shown. As in the case of Olsson et al. [78], statin trials occasionally provide unexpectedly low or high HDL-C responses. Milionis et al. [96] reported only a modest increase in HDL-C with rosuvastatin 10 mg (3.3%) and a fall of 1.6% with atorvastatin 20 mg. Negative HDL-C responses to pravastatin have been reported [34] as well as atypically low responses to starting doses of simvastatin [32, 38, 97–99]. In contrast, unexpected relatively large increases in HDL-C have occasionally been reported for pravastatin 10–40 mg [100–102], simvastatin [99, 103–105], and atorvastatin (7.3–9.0%) [105, 106]. In two studies of particular ethnic groups, one in African-Americans [94] and one in Hispanic-Americans [95], HDL-C responses to rosuvastatin and atorvastatin were consistent with Fig. 2a.
Most trials of the recommended starting doses of statins in hypercholesterolemic patients have reported positive effects on HDL, typically between 4% and 10% in terms of HDL-C. There was a tendency for rosuvastatin to provide greater increases than other statins. However, no consistent differences are apparent between the lower and higher starting dose of each statin.
5 Dose-related effects of statins on HDL up to the highest recommended doses
5.1 Statin dose–response
Figure 3 provides an overview of the five largest studies that measured the lipid effects of three or more doses of each statin. The variability in HDL-C and apo A-I responses in these trials was such that only if there had been gradients of at least 20% with each doubling of dose would clear dose–response relationships have been apparent. In the study of Jones et al. [72], for example, the coefficients of variation of percent change in HDL-C and apo A-I were approximately 200%. As evident in Fig. 3a, the confidence limits of the estimates of percent change in HDL-C overlap between doses in nearly all cases, making it uncertain whether or not true dose-relationships exist over the clinical range for each statin. The notable exception is atorvastatin, for which change in HDL-C can be seen to decrease from the lowest (10 mg) to the highest dose (80 mg) [72, 108, 109]. Between atorvastatin and rosuvastatin (the two statins with greatest effect on LDL-C), there are contrasting effects on HDL-C such that rosuvastatin maintains a relatively constant effect across doses whilst atorvastatin does not. Similar findings have been provided by smaller parallel group [29], forced-titration [110, 111] and crossover [112] trials. For simvastatin, even with an extended dose range of up to 160 mg, the effects on HDL parameters did not appear to be dose-related [112].
Dose-related effects of statins on a high-density lipoprotein cholesterol (HDL-C) and b apolipoprotein A-I (apo A-I) in hypercholesterolemic patients in five studies. Data are shown as mean percent change from baseline ±95% confidence limits when available. Dose (mg) is shown at the base of each column
The lack of obvious dose–response relationships has led to the suggestion that mechanisms different to those regulating the LDL response must predominate in mediating HDL changes [113]. However, as shown in Fig. 4, with the important exception of atorvastatin, the HDL-C changes observed by Jones et al. [72] do seem to parallel those of LDL-C and TG. In Fig. 4, the regression lines are fitted by robust MM-estimation that effectively down-weights outliers. Notably, the data points for atorvastatin deviate from the regression lines in a dose-related manner. It is perfectly possible that the effect of a statin on HDL parameters is the result of two or more competing processes such that any relationships with dose tend to be obscured. A tendency to increase HDL-C as a result of reduced CETP activity due to reductions in TG might be counteracted by negative effects due to depletion of hepatic cholesterol consequent on inhibition of HMG-CoA reductase. According to recent experimental work in animals, the liver could be the major source of the cholesterol that circulates in HDL [6]. It is also possible that such hypothetical negative effects might vary between different statins according to the unique pharmacodynamic profile of each compound.
Relationships between mean percent changes in high-density lipoprotein cholesterol (HDL-C) and mean percent changes in a triglycerides (TG) and b low-density lipoprotein cholesterol (LDL-C) in the STELLAR study [72]. Dashed lines calculated by a robust MM estimation. Panel a taken from Jones et al. [114]
This analysis suggests that with the exception of atorvastatin, statin-induced increases in HDL-C occur in parallel with reductions in apo B-containing lipoproteins, but that dose relationships can be obscured by high variability and competing effects. It is consistent however with the view that the predominant mechanism giving rise to statin-mediated increases in HDL-C is reduced cholesterol ester transfer into VLDL and LDL, secondary to reduced levels of these lipoproteins. The reason atorvastatin does not conform to this pattern and the potential clinical significance is a matter of current speculation.
5.2 Effects of statins at the maximal clinical doses
Figure 5 shows the HDL-C and apo A-I data from the five largest studies (three with HDL-C as the primary endpoint) employing the highest clinically-used doses of atorvastatin, simvastatin and rosuvastatin. All five studies reported a significantly lower HDL-C effect for atorvastatin compared with other statins. Crouse et al. [115] directly compared atorvastatin 20 mg with simvastatin 40 mg, and atorvastatin 40 mg with simvastatin 80 mg with the rationale that these particular statin/doses were approximately matched with respect to LDL-C reduction. Atorvastatin increased HDL-C significantly less than simvastatin in both comparisons, a finding confirmed by Kastelein et al. [116], and significant differences in apo A-I were also observed in the higher-dose comparative pair. In other randomized, double-blind trials of high-dose statin therapy, Smilde et al. [120] showed similar HDL-C increases for atorvastatin 80 mg and simvastatin 40 mg, while Ballantyne et al. [117] reported a significantly lower increase in HDL-C with atorvastatin 80 mg compared with simvastatin 80 mg, the effect of atorvastatin on apo A-I being slightly negative. Leiter et al. [119], compared rosuvastatin 40 mg with atorvastatin 80 mg, and found significantly greater increases in both HDL-C and apo A-I with rosuvastatin (9.6% and 4.2%, respectively) than atorvastatin (4.4% and 0.5%, respectively). Similar findings were reported in a forced-titration study of these two statins [121].
Effects of statins at the maximal clinical doses on a high-density lipoprotein cholesterol (HDL-C) and b apolipoprotein A-I (apo A-I) in hypercholesterolemic patients. Data shown as mean percent change from baseline ±95% confidence limits when available. Dose (mg) is shown given at the foot of each column
The percentage change in HDL-C induced by statins is usually greater in patients with lower than in those with higher baseline HDL-C levels. For example, Ballantyne et al. [117] reported greater HDL-C changes with baseline HDL-C <40 vs. ≥40 mg/dl when patients were treated with simvastatin 80 mg or atorvastatin 80 mg. Patients with acute coronary syndromes tend to have low HDL-C levels and in these, the HDL-C increases with statins were relatively high [122].
Comparisons between statins given at the higher doses consistently show atorvastatin to have a lesser effect on HDL compared to simvastatin or rosuvastatin, in line with the dose-related effects discussed earlier. Percentage changes in HDL-C are greater in patients with initially lower baseline values.
6 Effect of statins compared with other drugs that raise HDL
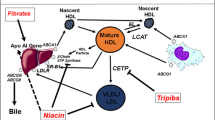
Fibrates are generally more effective than statins in lowering plasma TG and are used primarily in patients with elevated TG levels. Through their agonist action on PPARα, fibrates alter the expression and activity of genes involved in lipid metabolism, in particular increasing the hepatic synthesis of apo A-I [123]. Fibrates can reduce LDL, to a limited degree, but usually have a considerably greater effect on HDL than statins. This may be due both to the direct effect of increased entry of apo A-I into the circulation and to the indirect effect of reduction in VLDL-TG. For example, in a 24-week trial comparing gemfibrozil 600 mg twice daily with pravastatin 40 mg/d in patients with familial or polygenic hypercholesterolemia, TG <250 mg/dl and relatively normal HDL-C levels, pravastatin as expected lowered LDL-C more than gemfibrozil but had lesser effect on HDL-C (5% vs. 13%, respectively, p < 0.01), although changes in apo A-I were similar (8% vs. 7%, respectively) [124]. Similar differences were reported for these comparators by Wiklund et al. [35]. Superior effects on HDL-C favoring the fibrate have also been reported between gemfibrozil and simvastatin [125], bezafibrate and simvastatin [126], and micronized fenofibrate and pravastatin [127]. Two studies reported similar effects when comparing fenofibrate and simvastatin [128, 129]. The latter study found particularly high HDL-C responses (18% vs. 15%, respectively), the reason for which is unclear. Nicotinic acid (niacin) is a long-established therapy with modest effect on LDL but substantial impacts on TG and HDL. Depending upon the type of hyperlipidemia and baseline HDL-C, extended-release niacin can increase HDL-C by up to 30% and decrease TG levels by 40–50% [130, 131].
Unprecedented increases in HDL-C and apo A-I levels have been observed in phase II trials of the newly developed direct inhibitors of CETP. For example, in patients with low HDL-C levels, 4 weeks of treatment with torcetrapib 120 mg twice daily increased HDL-C from 34 to 70 mg/dl (106%) and apo A-I from 112 to 151 mg/dl (36%) [132], considerably greater increases than seen with statins, fibrates, or nicotinic acid. However, recent imaging and outcomes studies with torcetrapib in combination with atorvastatin have not indicated any beneficial impact upon atherosclerosis or cardiovascular morbidity and mortality, possibly due to a unique adverse effect of torcetrapib on aldosterone, electrolytes, and blood pressure [20–22, 133].
Fibrates and nicotinic acid increase HDL-C and lower TG usually to a greater extent than statins but their impact on LDL-C is considerably less. They are most useful in patients with hypertriglyceridemia. Direct inhibitors of CETP cause the greatest increases in HDL-C but the clinical value of this mechanism is currently unknown.
7 Effects of statins combined with other drugs on HDL in patients with hypercholesterolemia
Bile acid sequestrants such as cholestyramine and inhibitors of cholesterol absorption such as ezetimibe reduce levels of atherogenic lipoproteins albeit to a lesser degree than statins. Nevertheless, these agents can be effective in combination with statins, providing incremental reductions in LDL-C in cases of severe hypercholesterolemia and resistance to statin therapy. When used alone, these agents produce small increases (5–8%) in HDL-C [29, 30, 33, 103, 134]. However, while cholestyramine and pravastatin in combination provided a greater reduction in LDL-C than either monotherapy, there was no additive effect on HDL-C [33, 135]. Two 12-week studies compared the full range of simvastatin doses with and without ezetimibe 10 mg. The larger study [30] (n ≥ 156 per group) found no significant additive effect of ezetimibe on HDL-C whilst the smaller study [29] (n ≥ 52 per group) showed a modest but significant 2.4% (p < 0.05) increment averaged across doses. Similar rather modest incremental HDL-C effects of ezetimibe with statins were reported by Feldman et al. [136] and Ballantyne et al. [137, 138]. In these studies there was little evidence of any additive effects on apo A-I. Ballantyne et al. [108, 139] compared the full range of simvastatin doses in combination with ezetimibe to those of atorvastatin alone and found, at the 40 and 80 mg doses, a significantly greater effect on HDL-C with the simvastatin combination, which was attributed to the attenuation of the HDL-C response at higher doses described earlier. Catapano et al. [107] compared the ezetimibe/simvastatin combination in the range 10/20 to 10/80 mg with rosuvastatin 10/40 mg and found no difference in the HDL-C responses to those of the rosuvastatin monotherapy (7% to 8%). In summary, the addition of either bile acid sequestrants or inhibitors of cholesterol absorption provides at best only modest increments in the HDL-C response to statins.
8 Effects of statins in mixed hyperlipidemia, metabolic syndrome, and T2D
In many individuals, low HDL-C levels (<40 mg/dl men; <50 mg/dl women) are associated with elevated TG levels, which, in turn, are common in obesity, insulin resistance, metabolic syndrome, and T2D [140]. As noted above, there is a well-established inverse correlation between TG and HDL-C [56] and the combination of high TG, low HDL, and abnormal LDL (small-dense LDL), often called the “lipid triad”, is particularly prevalent in these conditions and thought to be strongly atherogenic [141]. Indeed, the linkage of HDL-C levels to these associated abnormalities of atherogenic lipoproteins may explain much of the predictive power of HDL-C.
8.1 Elevated triglycerides and mixed hyperlipidemia
Fibrates and nicotinic acid are the drugs of choice in severe hypertriglyceridemia. However, in mixed hyperlipidemia, when both TG and LDL are elevated, statins can provide a logical alternative as they are very effective in reducing LDL-C and can also lower TG. This point is supported by a number of studies [142–146]. The study by Grundy et al. [144] exemplifies the efficacy of a statin-fibrate combination in mixed hyperlipidemia. With simvastatin 20 mg together with fenofibrate 160 mg, HDL-C and apo A-I increased by 19% and 9% vs. 10% and 5% with statin alone. In a similar population, Farnier et al. [147] found that a combination of simvastatin 20 mg, fenofibrate 160 mg, and ezetimibe 10 mg raised HDL-C and apo A-I by 19% and 11% respectively. With both statins and fibrates, the increases in HDL-C generally reflect the reductions in VLDL-TG, suggesting reduced CETP-mediated flow of cholesteryl ester from HDL to VLDL as a major link between the HDL effects of statins and fibrates. Interestingly however, HDL size increased with simvastatin (3%) but not with gemfibrozil, hinting that there are differences as well as similarities in the mechanisms involved with the two classes of drugs.
8.2 Metabolic syndrome
Attention has increasingly focused on the effects of statins in ameliorating the high risk of CHD in patients with metabolic syndrome. Raised TG and low HDL-C represent two of five National Cholesterol Education Program (NCEP) criteria defining the metabolic syndrome [11]. In a placebo-controlled study of patients with metabolic syndrome so defined, atorvastatin 10 mg increased HDL-C by 5.1% and rosuvastatin 10 mg by 9.5% (p < 0.01 between treatments) compared with +1% for placebo [148]. There were corresponding but lesser changes in apo A-I. Similar increases in HDL-C have been seen in metabolic syndrome subgroups from larger trials [110, 149].
8.3 Diabetes
T2D carries a high risk of CHD. Statin therapy substantially reduces this risk and they are now the standard of care [150]. Evidence from trials of statins in this condition suggests that the degree of statin-induced change in HDL parameters depends on the baseline TG and HDL-C levels. For example, the study of Stein et al. [151] included patients with T2D and mixed hyperlipidemia having relatively high TG (median 391 mg/dl) and low HDL-C (mean 39 mg/dl) at baseline. After 6 weeks of treatment with simvastatin 40 or 80 mg/d, the increases in HDL-C were substantial: 13% and 16% compared to 3.3% with placebo; the corresponding changes in apo A-I were 8.2%, 10%, and 4.0%. In similar populations, Durrington et al. [152] observed that rosuvastatin 5 or 10 mg elevated HDL-C by 9.9% and 10% compared with 1.2% for placebo while Gentile et al. [153] recorded increments in HDL-C of 7.1–7.4% with atorvastatin, simvastatin, and pravastatin (each 10 mg/d) and a slight reduction with placebo. In T2D subjects with a baseline HDL-C <40 mg/dl (mean 34 mg/dl), Miller et al. [154] found HDL-C increases of 4.8% and 8.5% with simvastatin 40 and 80 mg, vs. a slight reduction with placebo. In contrast, in T2D study populations with relatively normal TG and HDL-C at baseline, the statin-induced HDL-C responses were usually relatively less as reported by Schweitzer et al. [155] and by Schuster et al. [68]. Moreover, in two large-scale outcome studies evaluating the effects of atorvastatin 10 mg in these patients, there were only non-significant changes in HDL-C (1–2%) and TG (−4% to −19%) [156, 157]. There has been only one study of patients with T2D that has recorded a substantial HDL-C response to atorvastatin (17%), but this compared to a 13% change with placebo [158]. In contrast to the many studies of statins in patients with T2D, relatively few have been performed in type 1 and none matched our selection criteria.
Statins are effective in raising HDL-C in mixed hyperlipidemia, metabolic syndrome, and T2D, but this seems to be dependent on baseline parameters. Statins are less effective than fibrates in raising HDL-C in these conditions but achieve much greater LDL-C reductions. The evidence suggests that combination of a statin with a fibrate may provide benefits both on LDL and on the TG-HDL axis.
9 Effects of statins on HDL over the longer-term and the relationship with clinical benefit
Data from placebo-controlled trials of long duration (Table 1) suggest that the effects of statins on HDL are maintained over time. The question of whether differential HDL effects between statins are also maintained is more difficult to answer, as few long-term trials have made such comparisons. However, the relatively attenuated HDL effect of high dose atorvastatin has been observed in long-term studies. In a double-blind comparison of atorvastatin 80 mg and pravastatin 40 mg [159], the percentage increases in HDL-C from baseline at 18 months were 2.9% and 5.6% respectively. An absolute difference in HDL-C levels of 1.2–1.9 mg/dl between the groups treated with simvastatin 20 mg or atorvastatin 80 mg (in favor of the former) was reported to be maintained over 4 years in the study of Pedersen et al. [160], although the difference diminished in the fifth year of observation. Similarly, in acute coronary syndrome patients, there was a difference of 1.6% in HDL-C levels between pravastatin 40 mg and atorvastatin 80 mg over a 2-year period, Cannon et al. [122]. However, there was no difference in this respect between 10 mg and 80 mg of atorvastatin over 5 years [66].
Statin treatment reduces coronary events in patients with low HDL-C levels [161–163]. Moreover, HDL-C levels continued to predict cardiovascular risk even when LDL-C was reduced to very low levels by atorvastatin [164]. However, the respective contribution made by statin-induced increases in HDL-C to cardiovascular benefit is not always apparent from clinical trial data. This may be due to considerable inter-individual differences (depending in turn on the particular combination of risk factors and genetic predisposition) in the degree to which a change in HDL impacts upon disease progression and perhaps most importantly to the overwhelming positive effect of reductions in LDL. In the outcome studies mentioned above in which statins were compared, the differences in HDL-C responses due to pravastatin in Cannon et al. [122] and simvastatin in Pedersen et al. [160] did not outweigh the superior LDL-C reductions due to atorvastatin 80 mg. The potential contributions made to outcomes by statin-induced changes in HDL-C are best discerned in the large scale such as in the Pravastatin Pooling Project, which combined data from three large trials of pravastatin 40 mg [162]. As expected, baseline LDL-C and HDL-C levels, analyzed in quintiles, were strongly predictive (positively and negatively, respectively) of events in the placebo group. In the treated group, the slope of the line relating baseline LDL-C to risk was markedly reduced, indicating the statin had markedly ameliorated LDL-associated risk. In contrast, the slope of the HDL-C line was only slightly reduced indicating a rather modest effect on the HDL-associated risk. This might be expected given that the effect of the statin on LDL was greater (−25% to −28%) than that on HDL-C (5%). Post hoc analysis of data from the Scandinavian Simvastatin Survival Study (4S) by Cox Proportional Hazard suggested an independent benefit of the simvastatin effect on HDL-C, a reduction in risk of 0.8% for each 1% increase [42, 161]. The application of other statistical models also found significant or marginally significant independent benefit arising from the increases in HDL-C. In an additional post hoc analysis of the 4S study, Ballantyne et al. [165] found that the subgroup defined as having HDL-C in the lowest and TG in the highest quartile had the most substantial event reduction.
In order to address this issue with respect to progression or regression of coronary atherosclerosis, Nicholls et al. [166] combined data from four trials that used intravascular ultrasound to measure atheroma volume. In multivariate analyses, as expected, the on-treatment LDL-C was correlated with the atheroma parameters, but the change in HDL-C (but not the on-treatment value) also made a strong independent contribution. Notably, participants who showed lesion regression manifested not only a low on-treatment LDL-C but also an HDL-C increase greater than the overall mean of 7.5%. There are some weaknesses in this post hoc analysis. For example in two of the pooled trials, statin therapy was unrandomized background treatment, all four trials were open-label and there were no placebo groups. Thus, while an association between change in HDL-C and reduced atherosclerosis progression was apparent, a direct, HDL-mediated causal effect of statins cannot be directly inferred. In a meta-analysis of 23 placebo-controlled trials with clinical cardiovascular endpoints, Brown et al. [167] found the best model to describe either change in stenosis or change in event rate involved the simple addition of percentage changes in LDL-C and HDL-C (R 2, 0.93 and 0.96, respectively), but it was not determined if the relationships between these two parameters and stenosis or risk were independent of one another.
With statins, the impact of reduction in the apo B-containing lipoproteins on atherosclerosis and cardiovascular events is sure to be greater than that due to any changes in HDL levels. Nevertheless, given the strength and independence of the epidemiologic relationships, it is reasonable to expect that statin-induced elevations in HDL make an independent contribution to benefit. It would be difficult to conceive a clinical outcomes trial that would provide absolute verification of this hypothesis. It would be necessary to compare statins in such a way that LDL-C levels were the same between groups (and equivalent in terms of other potential ‘pleiotropic’ effects), allowing only HDL-C responses to differ. However, as noted earlier, statins increase HDL parameters and lower TG, altering the HDL subpopulations in a way consistent with clinical benefit. The evidence related above lends some support to this hypothesis. Moreover, it is reasonable to expect that the degree of HDL-associated benefit of statins is proportional to the change in HDL levels. It is important therefore to assess statins not only in terms of their capacity to reduce LDL and VLDL but also their respective abilities to raise levels of HDL.
10 Summary and conclusions
The development of statins capable of profound reductions in the levels of apo B-containing atherogenic lipoproteins has been a major advance in cardiovascular medicine. In this respect, the statins have been thoroughly investigated and their within-class differences in, for example, the dose-related effects on LDL-C are well documented [72]. However, in view of the strong relationships between levels of HDL and cardiovascular risk, it is also important to understand the manner and degree of statin effects on these lipoproteins and whether there are important differences between them in this respect. However, most clinical trials of statins have not been set up with such aims in mind and have been underpowered with respect to HDL parameters. The purpose of this review has been to draw together in a systematic way all of the information about changes in HDL available from clinical trials that met certain predefined qualitative and quantitative criteria.
The data from placebo-controlled trials clearly demonstrated that statins induce real increases in both HDL-C and apo A-I. The increases in HDL-C were about twice those of apo A-I, consistent with specialized studies that have demonstrated alterations of HDL subpopulations towards larger more cholesterol-rich forms characteristic of healthy low-risk populations with low TG and high HDL-C. However, the changes in HDL parameters induced by statins are limited in comparison to fibrates and nicotinic acid. Percentage increases are usually greater in those with lower baseline levels. The data from the large morbidity and mortality trials suggested that the statin-induced effects on HDL-C and apo A-I were maintained over time. Moreover, analysis of the covariates of the HDL-C changes observed in these large studies suggested that CETP activity was a key factor that determines those changes. This makes it likely that the main mechanism of the elevations in HDL-C is a reduced rate of transfer of cholesterol from HDL to the apo B-containing lipoproteins consequent on the profound reductions in the latter. In hypercholesterolemic patient populations, the usual starting doses of statins provide increases in HDL-C of between 4% and 10%. There was a tendency for rosuvastatin to elevate HDL-C more than the other statins (atorvastatin, simvastatin, and pravastatin). In contrast to their effects on LDL-C and apo B, statins do not show clear-cut dose-response relationships with respect to HDL-C and apo A-I. However, further analysis of the largest dose–response study did indicate that the degree of change in HDL-C was partly related to the degree of reduction in TG and LDL-C, consistent with the suggested CETP-related mechanism. However, atorvastatin was a notable exception, in that there was an inverse dose-relationship with diminishing effects on HDL parameters with increasing dose. Comparisons at the highest clinical doses consistently showed atorvastatin to have the least effect on HDL parameters compared with the other three statins. In patients with mixed dyslipidemia, having high TG and low HDL-C as well as elevated LDL-C, statins induce more substantial percentage increases, and a combination of statin and fibrate provides optimal normalization of lipoprotein levels, including HDL.
The main cardiovascular benefits of statins are undoubtedly due to the major reductions of LDL and the other atherogenic lipoproteins. However, it is reasonable to suggest that increases in HDL may also make a valuable contribution to benefit since some of the data from the morbidity and mortality trials, as well as from imaging studies support this hypothesis. However, the independent contributions of the changes induced by statins on LDL and HDL remains an area that requires further study and clarification.
References
Miller NE, Thelle DS, Forde OH, Mjos OD. The Tromsø Heart-Study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet 1977;1:965–8.
Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124:S11–S20.
Gordon DJ. Factors affecting high-density lipoproteins. Endocrinol Metab Clin N Am 1998;27:699–709 (xi).
Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 2001;358:2026–33.
Von Eckardstein A, Langer C, Engel T, et al. ATP binding cassette transporter ABCA1 modulates the secretion of apolipoprotein E from human monocyte-derived macrophages. FASEB J 2001;15:1555–61.
Brewer HB Jr, Remaley AT, Neufeld EB, Basso F, Joyce C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol 2004;24:1755–60.
Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis 2002;161:1–16.
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res 2004;95:764–72.
Qiao Q, Gao W, Zhang L, Nyamdorj R, Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem 2007;44:232–63.
Sviridov D, Nestel PJ. Genetic factors affecting HDL levels, structure, metabolism and function. Curr Opin Lipidol 2007;18:157–63.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators, Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78.
Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8–15.
Barter PJ. Options for therapeutic intervention: how effective are the different agents? Eur Heart J Suppl 2006;8:F47–F53.
Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA 1988;260:641–51.
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–8.
The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360–81.
Brown WV. What are the priorities for managing cholesterol effectively? Am J Cardiol 2001;88:21F–24F.
Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292–300.
Nissen SE, Tardif JC, Nicholls SJ, et al. ILLUSTRATE Investigators. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304–16.
Kastelein JJ, van Leuven SI, Burgess L, et al. RADIANCE 1 Investigators. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620–30.
Bots ML, Visseren FL, Evans GW, et al. RADIANCE 2 Investigators. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 2007;370:153–60.
Martin G, Duez H, Blanquart C, et al. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J Clin Invest 2001;107:1423–32.
Guerin M, Lassel TS, Le Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler Thromb Vasc Biol 2000;20:189–97.
Warnick GR, Nauck M, Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin Chem 2001;47:1579–96.
Hunninghake DB, Knopp RH, Schonfeld G, et al. Efficacy and safety of pravastatin in patients with primary hypercholesterolemia. I. A dose–response study. Atherosclerosis 1990;85:81–9.
Davidson M, McKenney J, Stein E, et al. Comparison of one-year efficacy and safety of atorvastatin versus lovastatin in primary hypercholesterolemia. Atorvastatin Study Group I. Am J Cardiol 1997;79:1475–81.
Davidson M, Ma P, Stein EA, et al. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am J Cardiol 2002;89:268–75.
Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol 2002;40:2125–34.
Bays HE, Ose L, Fraser N, et al. Ezetimibe Study Group. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther 2004;26:1758–73.
The Pravastatin Multinational Study Group for Cardiac Risk Patients. Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. Am J Cardiol 1993;72:1031–7.
Haffner S, Orchard T, Stein E, Schmidt D, LaBelle P. Effect of simvastatin on Lp(a) concentrations. Clin Cardiol 1995;18:261–7.
Pravastatin Multicenter Study Group II. Comparative efficacy and safety of pravastatin and cholestyramine alone and combined in patients with hypercholesterolemia. Arch Intern Med 1993;153:1321–9.
Rosenson RS, Bays HE. Results of two clinical trials on the safety and efficacy of pravastatin 80 and 160 mg per day. Am J Cardiol 2003;91:878–81.
Wiklund O, Angelin B, Bergman M, et al. Pravastatin and gemfibrozil alone and in combination for the treatment of hypercholesterolemia. Am J Med 1993;94:13–20.
Bak AA, Huizer J, Leijten PA, Rila H, Grobbee DE. Diet and pravastatin in moderate hypercholesterolaemia: a randomized trial in 215 middle-aged men free from cardiovascular disease. J Intern Med 1998;244:371–8.
Capurso A, Resta F, Bertolini S, et al. Lipid control with low-dosage simvastatin in patients with moderate hypercholesterolaemia. An Italian multicentre double-blind placebo-controlled study. Eur Heart J 1992;13:11–6.
de Jongh S, Ose L, Szamosi T, et al. Simvastatin in Children Study Group. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation 2002;106:2231–7.
Frederiksen SM, Larsen ML, Oxenbøll IB, Pindborg T, Haghfelt T. Treatment of primary hypercholesterolemia with pravastatin. A placebo-controlled trial. Ugeskr Laeger 1993;155:2794–9.
Jacobson TA, Chin MM, Curry CL, et al. Efficacy and safety of pravastatin in African Americans with primary hypercholesterolemia. Arch Intern Med 1995;155:1900–6.
Keech A, Collins R, MacMahon S, et al. Three-year follow-up of the Oxford Cholesterol Study: assessment of the efficacy and safety of simvastatin in preparation for a large mortality study. Eur Heart J 1994;15:255–69.
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9.
Salonen R, Nyyssönen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92:1758–64.
Pitt B, Mancini GB, Ellis SG, Rosman HS, Park JS, McGovern ME. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC I): reduction in atherosclerosis progression and clinical events. PLAC I investigation. J Am Coll Cardiol 1995;26:1133–9.
Byington RP, Furberg CD, Crouse JR 3rd, Espeland MA, Bond MG. Pravastatin, lipids, and atherosclerosis in the carotid arteries (PLAC-II). Am J Cardiol 1995;76:54C–9C.
Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The regression growth evaluation statin study (REGRESS). Circulation 1995;91:2528–40.
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333:1301–7.
Mercuri M, Bond MG, Sirtori CR, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic Mediterranean population: the carotid atherosclerosis Italian ultrasound study. Am J Med 1996;101:627–34.
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med 1996;335:1001–9.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
Tonkin AM, Colquhoun D, Emberson J, et al. Effects of pravastatin in 3260 patients with unstable angina: results from the LIPID Study. Lancet 2000;356:1871–5.
Sever PS, Dahlöf B, Poulter NR, et al. ASCOT Investigators. prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
Crouse JR 3rd, Raichlen JS, Riley WA, et al. METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 2007;297:1344–53.
Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–61.
Lamarche B, Després JP, Moorjani S, Cantin B, Dagenais GR, Lupien PJ. Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease. Results from the Québec Cardiovascular Study. Atherosclerosis 1996;119:235–45.
Streja L, Packard CJ, Shepherd J, Cobbe S, Ford I. WOSCOPS Group. Factors affecting low-density lipoprotein and high-density lipoprotein cholesterol response to pravastatin in the West of Scotland Coronary Prevention Study (WOSCOPS). Am J Cardiol 2002;90:731–6.
Asztalos BF, Roheim PS, Milani RL, et al. Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 2000;20:2670–6.
Asztalos BF. HDL Atherosclerosis Treatment Study. High-density lipoprotein metabolism and progression of atherosclerosis: new insights from the HDL Atherosclerosis Treatment Study. Curr Opin Cardiol 2004;19:385–91.
Asztalos BF, Collins D, Cupples LA, et al. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol 2005;25:2185–91.
Asztalos BF, Le Maulf F, Dallal GE, et al. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high density lipoproteins. Am J Cardiol 2007;99:681–5.
Zambon A, Austin MA, Brown BG, et al. Effect of hepatic lipase on LDL in normal men and those with coronary heart disease. Arterioscler Thromb 1993;13:147–53.
Watson TDG, Caslake MJ, Freeman DJ, et al. Determinants of LDL subfraction distribution and concentrations in young normolipidemic subjects. Arterioscler Thromb 1994;14:902–10.
Kuusi T, Saarinen P, Nikkila EA. Evidence for the role of hepatic endothelial lipase in the metabolism of plasma high density lipoprotein2 in man. Atherosclerosis 1980;36:589–93.
Zambon A, Deeb SS, Brown BG, Hokanson JE, Brunzell JD. Common hepatic lipase gene promoter variant determines clinical response to intensive lipid-lowering treatment. Circulation 2001;103:792–8.
LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J 2001;22:554–72.
Schuster H, Barter PJ, Stender S, et al. Effective reductions in cholesterol using rosuvastatin therapy i study group. Effects of switching statins on achievement of lipid goals: measuring effective reductions in cholesterol using rosuvastatin therapy (MERCURY I) study. Am Heart J 2004;147:705–13.
Cheung RC, Morrell JM, Kallend D, Watkins C, Schuster H. Effects of switching statins on lipid and apolipoprotein ratios in the MERCURY I study. Int J Cardiol 2005;100:309–16.
Ballantyne CM, Bertolami M, Hernandez Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol, and apolipoprotein B target levels in high-risk patients: measuring effective reductions in cholesterol using rosuvastatin therapy (MERCURY) II. Am Heart J 2006;151:975, e1–9.
Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and special population groups. Am J Cardiol 2003;91:3C–10C.
Jones PH, Davidson MH, Stein EA, et al. STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 2003;92:152–60.
Jones PH, Hunninghake DB, Ferdinand KC, et al. STELLAR Study Group. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther 2004;26:1388–99.
Schwartz GG, Bolognese MA, Tremblay BP, et al. Efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: a randomized, controlled trial. Am Heart J 2004;148:e4.
Olsson AG, Istad H, Luurila O, et al. Rosuvastatin investigators group. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. Am Heart J 2002;144:1044–51.
Paoletti R, Fahmy M, Mahla G, Mizan J, Southworth H. Rosuvastatin demonstrates greater reduction of low-density lipoprotein cholesterol compared with pravastatin and simvastatin in hypercholesterolaemic patients: a randomized, double-blind study. J Cardiovasc Risk 2001;8:383–90.
Brown WV, Bays HE, Hassman DR, et al. Rosuvastatin study group. Efficacy and safety of rosuvastatin compared with pravastatin and simvastatin in patients with hypercholesterolemia: a randomized, double-blind, 52-week trial. Am Heart J 2002;144:1036–43.
Olsson AG, Eriksson M, Johnson O, et al. 3T Study Investigators. A 52-week, multicenter, randomized, parallel-group, double-blind, double-dummy study to assess the efficacy of atorvastatin and simvastatin in reaching low-density lipoprotein cholesterol and triglyceride targets: the treat-to-target (3T) study. Clin Ther 2003;25:119–38.
Barter PJ, O’Brien RC. Achievement of target plasma cholesterol levels in hypercholesterolaemic patients being treated in general practice. Atherosclerosis 2000;149:199–205.
Insull W Jr, Ghali JK, Hassman DR, et al. SOLAR Study Group. Achieving low-density lipoprotein cholesterol goals in high-risk patients in managed care: comparison of rosuvastatin, atorvastatin, and simvastatin in the SOLAR trial. Mayo Clin Proc 2007;82:543–50.
Bays HE, Dujovne CA, McGovern ME, et al. ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation, Comparison of once-daily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]). Am J Cardiol 2003;91:667–72.
Dujovne CA, Knopp R, Kwiterovich P, Hunninghake D, McBride TA, Poland M. Randomized comparison of the efficacy and safety of cerivastatin and pravastatin in 1,030 hypercholesterolemic patients. The Cerivastatin Study Group. Mayo Clin Proc 2000;75:1124–32.
Farmer JA, Washington LC, Jones PH, Shapiro DR, Gotto AM Jr, Mantell G. Comparative effects of simvastatin and lovastatin in patients with hypercholesterolemia. The Simvastatin and Lovastatin Multicenter Study Participants. Clin Ther 1992;14:708–17.
Saunders E, Ferdinand K, Yellen LG, Tonkon MJ, Krug-Gourley S, Poland M. Efficacy and safety of cerivastatin and pravastatin in the treatment of primary hypercholesterolemia. J Natl Med Assoc 2000;92:319–26.
The Simvastatin Pravastatin Study Group. Comparison of the efficacy, safety and tolerability of simvastatin and pravastatin for hypercholesterolemia. Am J Cardiol 1993;71:1408–14.
Steinhagen-Thiessen E. Comparative efficacy and tolerability of 5 and 10 mg simvastatin and 10 mg pravastatin in moderate primary hypercholesterolemia. Simvastatin Pravastatin European Study Group. Cardiology 1994;85:244–54.
Weir MR, Berger ML, Weeks ML, Liss CL, Santanello NC. Comparison of the effects on quality of life and of the efficacy and tolerability of lovastatin versus pravastatin. The quality of life multicenter group. Am J Cardiol 1996;77:475–9.
Bertolini S, Bon GB, Campbell LM, et al. Efficacy and safety of atorvastatin compared to pravastatin in patients with hypercholesterolemia. Atherosclerosis 1997;130:191–7.
The European Study Group. Efficacy and tolerability of simvastatin and pravastatin in patients with primary hypercholesterolemia (multicountry comparative study). Am J Cardiol 1992;70:1281–6.
Hunninghake D, Bakker-Arkema RG, Wigand JP, et al. Treating to meet NCEP-recommended LDL cholesterol concentrations with atorvastatin, fluvastatin, lovastatin, or simvastatin in patients with risk factors for coronary heart disease. J Fam Pract 1998;47:349–56.
Lambrecht LJ, Malini PL. Efficacy and tolerability of simvastatin 20 mg vs pravastatin 20 mg in patients with primary hypercholesterolemia. European Study Group. Acta Cardiol 1993;48:541–54.
Malini PL, Ambrosioni E, De Divitiis O, Di Somma S, Rosiello G, Trimarco B. Simvastatin versus pravastatin: efficacy and tolerability in patients with primary hypercholesterolemia. Clin Ther 1991;13:500–10.
Wu CC, Sy R, Tanphaichitr V, Hin AT, Suyono S, Lee YT. Comparing the efficacy and safety of atorvastatin and simvastatin in Asians with elevated low-density lipoprotein-cholesterol—a multinational, multicenter, double-blind study. J Formos Med Assoc 2002;101:478–87.
Ferdinand KC, Clark LT, Watson KE, et al. ARIES Study Group. Comparison of efficacy and safety of rosuvastatin versus atorvastatin in African-American patients in a six-week trial. Am J Cardiol 2006;97:229–35.
Lloret R, Ycas J, Stein M, Haffner S. STARSHIP Study Group. Comparison of rosuvastatin versus atorvastatin in Hispanic-Americans with hypercholesterolemia (from the STARSHIP Trial). Am J Cardiol 2006;98:768–73.
Milionis HJ, Rizos E, Kostapanos M, et al. Treating to target patients with primary hyperlipidaemia: comparison of the effects of ATOrvastatin and ROSuvastatin (the ATOROS Study). Curr Med Res Opin 2006;22:1123–31.
Farnier M, Portal JJ, Maigret P. Efficacy of atorvastatin compared with simvastatin in patients with hypercholesterolemia. J Cardiovasc Pharmacol Ther 2000;5:27–32.
Milionis HJ, Kakafika AI, Tsouli SG, et al. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. Am Heart J 2004;148:635–40.
Simons LA. Comparison of atorvastatin alone versus simvastatin +/− cholestyramine in the management of severe primary hypercholesterolaemia (The Six Cities Study). Aust N Z J Med 1998;28:327–33.
The Lovastatin Pravastatin Study Group. A multicenter comparative trial of lovastatin and pravastatin in the treatment of hypercholesterolemia. Am J Cardiol. 1993;71:810–5.
Jacotot B, Benghozi R, Pfister P, Holmes D. Comparison of fluvastatin versus pravastatin treatment of primary hypercholesterolemia. French Fluvastatin Study Group. Am J Cardiol 1995;76:54A–6A.
McPherson R, Bedard J, Connelly P, et al. Comparison of the short-term efficacy and tolerability of lovastatin and pravastatin in the management of primary hypercholesterolemia. Clin Ther 1992;14:276–91.
Deslypere JP. Comparison between low-dose simvastatin and cholestyramine in moderately severe hypercholesterolemia. Acta Cardiol 1989;44:379–88.
Pietro DA, Alexander S, Mantell G, Staggers JE, Cook TJ. Effects of simvastatin and probucol in hypercholesterolemia (Simvastatin Multicenter Study Group II). Am J Cardiol 1989;63:682–6.
Illingworth DR, Crouse JR 3rd, Hunninghake DB, Simvastatin Atorvastatin HDL Study Group, et al. A comparison of simvastatin and atorvastatin up to maximal recommended doses in a large multicenter randomized clinical trial. Curr Med Res Opin 2001;17:43–50.
Brown AS, Bakker-Arkema RG, Yellen L, et al. Treating patients with documented atherosclerosis to National Cholesterol Education Program-recommended low-density-lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. J Am Coll Cardiol 1998;32:665–72.
Catapano AL, Davidson MH, Ballantyne CM, et al. Lipid-altering efficacy of the ezetimibe/simvastatin single tablet versus rosuvastatin in hypercholesterolemic patients. Curr Med Res Opin 2006;22:2041–53.
Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) Study. Am Heart J 2005;149:464–73.
Jones PH, McKenney JM, Karalis DG, Downey J. Comparison of the efficacy and safety of atorvastatin initiated at different starting doses in patients with dyslipidemia. Am Heart J 2005;149:e1.
Hunninghake DB, Ballantyne CM, Maccubbin DL, Shah AK, Gumbiner B, Mitchel YB. Comparative effects of simvastatin and atorvastatin in hypercholesterolemic patients with characteristics of metabolic syndrome. Clin Ther 2003;25:1670–86.
Stein EA, Strutt K, Southworth H, Diggle PJ, Miller E. HeFH Study Group. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. Am J Cardiol 2003;92:1287–93.
Davidson MH, Stein EA, Dujovne CA, et al. The efficacy and six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol 1997;79:38–42.
Barter PJ, Brewer HB Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160–7.
Jones PH, McTaggart F, Southworth H. Statin effects on high-density lipoprotein levels have both triglyceride-dependent and independent components. Atheroscler Suppl 2006;7:577–8. (Abstract Th-P16:383).
Crouse JR 3rd, Frohlich J, Ose L, Mercuri M, Tobert JA. Effects of high doses of simvastatin and atorvastatin on high-density lipoprotein cholesterol and apolipoprotein A-I. Am J Cardiol 1999;83:1476–7.
Kastelein JJ, Isaacsohn JL, Ose L, et al. Comparison of effects of simvastatin versus atorvastatin on high-density lipoprotein cholesterol and apolipoprotein A-I levels. Am J Cardiol 2000;86:221–3.
Ballantyne CM, Blazing MA, Hunninghake DB, et al. Effect on high-density lipoprotein cholesterol of maximum dose simvastatin and atorvastatin in patients with hypercholesterolemia: results of the Comparative HDL Efficacy and Safety Study (CHESS). Am Heart J 2003;146:862–9.
Karalis DG, Ross AM, Vacari RM, Zarren H, Scott R. Comparison of efficacy and safety of atorvastatin and simvastatin in patients with dyslipidemia with and without coronary heart disease. Am J Cardiol 2002;89:667–71.
Leiter LA, Rosenson RS, Stein E, POLARIS study investigators, et al. Efficacy and safety of rosuvastatin 40 mg versus atorvastatin 80 mg in high-risk patients with hypercholesterolemia: results of the POLARIS study. Atherosclerosis 2007;194:e154–e64.
Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001;357:577–81.
Faergeman O, Sosef F, Duffield E, On behalf of the ECLIPSE Study Investigators. Efficacy and tolerability of rosuvastatin and atorvastatin when force-titrated in high-risk patients: results from the ECLIPSE Study. Atheroscler Suppl 2006;7:580. (Abstract Th-P16:394).
Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. (erratum in: N Engl J Med 2006;354:778).
Duez H, Lefebvre B, Poulain P, et al. Regulation of human apoA-I by gemfibrozil and fenofibrate through selective peroxisome proliferator-activated receptor alpha modulation. Arterioscler Thromb Vasc Biol 2005;25:585–91.
Crepaldi G, Baggio G, Arca M, et al. Pravastatin vs gemfibrozil in the treatment of primary hypercholesterolemia. The Italian Multicenter Pravastatin Study I. Arch Intern Med 1991;151:146–52.
Tikkanen MJ, Bocanegra TS, Walker JF, Cook T. Comparison of low-dose simvastatin and gemfibrozil in the treatment of elevated plasma cholesterol. A multicenter study. The Simvastatin Study Group. Am J Med 1989;87:47S–53S.
Nakandakare E, Garcia RC, Rocha JC, et al. Effects of simvastatin, bezafibrate and gemfibrozil on the quantity and composition of plasma lipoproteins. Atherosclerosis 1990;85:211–7.
Ducobu J, VanHaelst L, Salomon H. Comparison of micronized fenofibrate and pravastatin in patients with primary hyperlipidemia. J Cardiovasc Pharmacol 2003;41:60–7.
Ziegler O, Drouin P. Safety, tolerability, and efficacy of simvastatin and fenofibrate—a multicenter study. Simvastatin–Fenofibrate Study Group. Cardiology 1990;77:50–7.
Steinmetz A, Schwartz T, Hehnke U, Kaffarnik H. Multicenter comparison of micronized fenofibrate and simvastatin in patients with primary type IIA or IIB hyperlipoproteinemia. J Cardiovasc Pharmacol 1996;27:563–70.
Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Increasing HDL cholesterol with extended-release nicotinic acid: from promise to practice. Neth J Med 2004;62:229–34.
Singh U, Otvos J, Dasgupta A, de Lemos JA, Devaraj S, Jialal I. High-dose alpha-tocopherol therapy does not affect HDL subfractions in patients with coronary artery disease on statin therapy. Clin Chem 2007;53:525–8.
Brousseau ME, Schaefer EJ, Wolfe ML, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med 2004;350:1505–15.
Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22.
Stein E, Kreisberg R, Miller V, Mantell G, Washington L, Shapiro DR. Effects of simvastatin and cholestyramine in familial and nonfamilial hypercholesterolemia. Multicenter Group I. Arch Intern Med 1990;150:341–5.
Ballantyne CM, Miller E, Chitra R. Efficacy and safety of rosuvastatin alone and in combination with cholestyramine in patients with severe hypercholesterolemia: a randomized, open-label, multicenter trial. Clin Ther 2004;26:1855–64.
Feldman T, Koren M, Insull W Jr, et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol 2004;93:1481–6.
Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol 2004;93:1487–94.
Ballantyne CM, Weiss R, Moccetti T, et al. EXPLORER Study Investigators. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol 2007;99:673–80.
Ballantyne CM, Houri J, Notarbartolo A, et al. Ezetimibe Study Group. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003;107:2409–15.
Després JP, Lemieux I, Dagenais GR, Cantin B, Lamarche B. HDL-cholesterol as a marker of coronary heart disease risk: the Québec Cardiovascular Study. Atherosclerosis 2000;153:263–72.
Nesto RW. Beyond low-density lipoprotein: addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am J Cardiovasc Drugs 2005;5:379–87.
Fogari R, Marasi G, Vanasia A, Zoppi A, Lusardi P, Preti P. Comparative study of acipimox and pravastatin in patients with combined hyperlipidemia. Int J Clin Pharmacol Ther 1997;35:61–4.
Insull W, Kafonek S, Goldner D, Zieve F. Comparison of efficacy and safety of atorvastatin (10 mg) with simvastatin (10 mg) at six weeks. ASSET Investigators. Am J Cardiol 2001;87:554–9.
Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am J Cardiol 2005;95:462–8. (erratum in: Am J Cardiol 2006;96:427–8).
Nestel P, Simons L, Barter P, et al. A comparative study of the efficacy of simvastatin and gemfibrozil in combined hyperlipoproteinemia: prediction of response by baseline lipids, apo E genotype, lipoprotein(a) and insulin. Atherosclerosis 1997;129:231–9.
McKenney JM, McCormick LS, Weiss S, Koren M, Kafonek S, Black DM. A randomized trial of the effects of atorvastatin and niacin in patients with combined hyperlipidemia or isolated hypertriglyceridemia. Collaborative Atorvastatin Study Group. Am J Med 1998;104:137–43.
Farnier M, Roth E, Gil-Extremera B, Ezetimibe/Simvastatin + Fenofibrate Study Group, et al. Efficacy and safety of the coadministration of ezetimibe/simvastatin with fenofibrate in patients with mixed hyperlipidaemia. Am Heart J 2007;153:335. (e1–e8).
Stalenhoef AF, Ballantyne CM, Sarti C, et al. A comparative study with rosuvastatin in subjects with the metabolic syndrome: results of the COMETS study. Eur Heart J 2005;26:2664–72.
Deedwania PC, Hunninghake DB, Bays HE, et al. STELLAR Study Group, Effects of rosuvastatin, atorvastatin, simvastatin, and pravastatin on atherogenic dyslipidemia in patients with characteristics of the metabolic syndrome. Am J Cardiol 2005;95:360–6.
Neil HA, DeMicco DA, Luo D, et al. CARDS Study Investigators, Analysis of efficacy and safety in patients aged 65–75 years at randomization: Collaborative Atorvastatin Diabetes Study (CARDS). Diabetes Care 2006;29:2378–84.
Stein E, Plotkin D, Bays H, et al. Effects of simvastatin (40 and 80 mg/day) in patients with mixed hyperlipidemia. Am J Cardiol 2000;86:406–11.
Durrington PN, Tuomilehto J, Hamann A, Kallend D, Smith K. Rosuvastatin and fenofibrate alone and in combination in type 2 diabetes patients with combined hyperlipidaemia. Diabetes Res Clin Pract 2004;64:137–51.
Gentile S, Turco S, Guarino G, et al. Comparative efficacy study of atorvastatin vs simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia. Diabetes Obes Metab 2000;2:355–62.
Miller M, Dobs A, Yuan Z, Battisti WP, Borisute H, Palmisano J. Effectiveness of simvastatin therapy in raising HDL-C in patients with type 2 diabetes and low HDL-C. Curr Med Res Opin 2004;20:1087–94.
Schweitzer M, Tessier D, Vlahos WD, et al. A comparison of pravastatin and gemfibrozil in the treatment of dyslipoproteinemia in patients with non-insulin-dependent diabetes mellitus. Atherosclerosis 2002;162:201–10.
Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS Investigators, Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
Knopp RH, d’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006;29:1478–85.
Soedamah-Muthu SS, Colhoun HM, Thomason MJ, et al. CARDS Investigators, The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis 2003;167:243–55.
Nissen SE, Tuzcu EM, Schoenhagen P, et al. REVERSAL Investigators, Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071–80.
Pedersen TR, Faergeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group., High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294:2437–45.
Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998;97:1453–60.
Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation 2000;102:1893–900.
Gotto AM Jr, Whitney E, Stein EA, et al. Relation between baseline and on-treatment lipid parameters and first acute major coronary events in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Circulation 2000;101:477–84.
Barter P, Gotto AM, LaRosa JC, et al. Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301–10.
Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation 2001;104:3046–51.
Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007;297:499–508.
Brown BG, Stukovsky KH, Zhao XQ. Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: a meta-analysis of 23 randomized lipid trials. Curr Opin Lipidol 2006;17:631–6.
Acknowledgements
We thank Catherine Harmston and Carl Felton, from Prime Medica Ltd., who provided medical writing support funded by AstraZeneca.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
McTaggart, F., Jones, P. Effects of Statins on High-Density Lipoproteins: A Potential Contribution to Cardiovascular Benefit. Cardiovasc Drugs Ther 22, 321–338 (2008). https://doi.org/10.1007/s10557-008-6113-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-008-6113-z