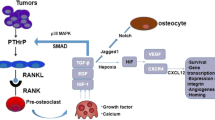
Abstract
The treatment of bone-metastatic cancer now takes advantage of the unique biology of this clinical state. The complex interplay between the cancer cells and the bone microenvironment leads to a host of therapeutic targets, with agents in various stages of clinical use or study. Targets include interactions between the cancer cells and osteoclasts, osteoblasts, endothelial cells, stromal cells, hematopoietic progenitor cells, cells of the immune system, and the bone matrix. Efforts at understanding specific mechanisms of drug resistance in the bone are also ongoing. Successful clinical outcomes will be the result of co-targeting and interrupting the various tumor-supportive elements and cooperating pathways at the level of the tumor cell, the primary and metastatic microenvironments, and systemic cancer effects, leading to a “scaled network disruption” to undermine the disease state.


Similar content being viewed by others
References
Li, S., Peng, Y., Weinhandl, E. D., Blaes, A. H., Cetin, K., & Chia, V. M., et al. (2012). Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol, 4, 87–93.
Loberg, R. D., Bradley, D. A., Tomlins, S. A., Chinnaiyan, A. M., & Pienta, K. J. (2007). The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA: A Cancer Journal for Clinicians, 57(4), 225–241.
Fidler, I. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews. Cancer, 3, 1–6.
Paget, S. (1889). The distribution of secondary growth in cancer of breast. Lancet, 1, 98–101.
Ewing, J. (1919). Metastasis. In Neoplastic diseases (pp. 76–88). Philadelphia: Saunders.
Pienta, K. J., & Loberg, R. (2005). The ‘emigration, migration, and immigration’ of prostate cancer. Clinical Prostate Cancer, 4(1), 24–30.
Norton, L. (1988). A Gompertzian model of human breast cancer growth. Cancer Research, 48, 7067–7071.
Zetter, B. (1998). Angiogenesis and tumor metastasis. Annual Review of Medicine, 49, 407–424.
Wang, J., Loberg, R., & Taichman, R. S. (2006). The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Reviews, 25(4), 573–587.
Kortesidis, A., Zannettino, A., Isenmann, S., Shi, S., Lapidot, T., & Gronthos, S. (2005). Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood, 105(10), 3793–3801.
Jung, Y., Wang, J., Schneider, A., Sun, Y.-X., Koh-Paige, A. J., Osman, N. I., et al. (2006). Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone, 38(4), 497–508.
Sun, Y., Fang, M., Wang, J., Cooper, C. R., Pienta, K. J., & Taichman, R. S., et al. (2007). Expression and Activation of a v b 3 Integrins by SDF-1 / CXC12 Increases the Aggressiveness of Prostate Cancer Cells. Prostate, 67(1), 61–73.
Sun, Y.-X., Schneider, A., Jung, Y., Wang, J., Dai, J., Wang, J., et al. (2005). Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of Bone and Mineral Research, 20(2), 318–329.
Havens, A. M., Jung, Y., Sun, Y. X., Wang, J., Shah, R., Bühring, H., et al. (2006). The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC cancer, 6, 195.
Jung, Y., Wang, J., Song, J., Shiozawa, Y., Wang, J., Havens, A., et al. (2007). Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood, 110(1), 82–90.
Sikes, R. A., Nicholson, B. E., Koeneman, K. S., Edlund, N. M., Bissonette, E. A., Bradley, M. J., et al. (2004). Cellular interactions in the tropism of prostate cancer to bone. International Journal of Cancer, 110(4), 497–503.
Chung, L. W. K., Baseman, A., Assikis, V., & Zhau, H. E. (2005). Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. The Journal of Urology, 173, 10–20.
Lipton, A. (2006). Future treatment of bone metastases. Clinical Cancer Research, 12(20 Pt 2), 6305s–6308s.
Mundy, G. R. (2002). Metastasis to bone: causes, consequences and therapeutic opportunities. Nature reviews. Cancer, 2(8), 584–593.
Loberg, R. D., Logothetis, C. J., Keller, E. T., & Pienta, K. J. (2005). Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 23(32), 8232–8241.
Candelaria-Quintana, D., Dayao, Z. R., & Royce, M. E. (2012). The role of antiresorptive therapies in improving patient care in early and metastatic breast cancer. Breast cancer research and treatment, 132(2), 355–363.
Fornier, M. N. (2010). Denosumab: second chapter in controlling bone metastases or a new book? Journal of Clinical Oncology, 28(35), 5127–5131.
Ha, T. C., & Li, H. (2007). Meta-analysis of clodronate and breast cancer survival. British Journal of Cancer, 96(12), 1796–1801.
Wu, S., Dahut, W. L., & Gulley, J. L. (2007). The use of bisphosphonates in cancer patients. Acta Oncologica, 46(5), 581–591.
Saad, F., Gleason, D. M., Murray, R., Tchekmedyian, S., Venner, P., Lacombe, L., et al. (2004). Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. Journal of the National Cancer Institute, 96(11), 879–882.
Lüftner, D., Henschke, P., & Possinger, K. (2007). Clinical value of bisphosphonates in cancer therapy. Anticancer research, 27(4A), 1759–1768.
Schwarz, E. M., & Ritchlin, C. T. (2007). Clinical development of anti-RANKL therapy. Arthritis Research & Therapy, 9(Suppl 1), S7.
Tsourdi, E., Rachner, T. D., Rauner, M., Hamann, C., & Hofbauer, L. C. (2011). Denosumab for bone diseases: translating bone biology into targeted therapy. European Journal of Endocrinology, 165(6), 833–840.
Miyazaki, T., Tanaka, S., Sanjay, A., & Baron, R. (2006). The role of c-Src kinase in the regulation of osteoclast function. Modern Rheumatology, 16(2), 68–74.
Huang, F., Reeves, K., Han, X., Fairchild, C., Platero, S., Wong, T. W., et al. (2007). Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer research, 67(5), 2226–2238.
Vandyke, K., Dewar, A. L., Diamond, P., Fitter, S., Schultz, C. G., Sims, N. A., et al. (2010). The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. Journal of Bone and Mineral Research, 25(8), 1759–1770.
Ritchie, C. K., Andrews, L. R., Thomas, K. G., Tindall, D. J., & Fitzpatrick, L. A. (1997). The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis : implications for the development of metastatic disease. Endocrinology, 138(3), 1145–1150.
Zangari, M., Esseltine, D., Lee, C.-K., Barlogie, B., Elice, F., Burns, M. J., et al. (2005). Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. British Journal of Haematology, 131, 71–73.
Terpos, E., Heath, D. J., Rahemtulla, A., Zervas, K., Chantry, A., Anagnostopoulos, A., et al. (2006). Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. British Journal of Haematology, 135(5), 688–692.
Vessella, R. L., & Corey, E. (2006). Targeting factors involved in bone remodeling as treatment strategies in prostate cancer bone metastasis. Clinical Cancer Research, 12(20 Pt 2), 6285s–6290s.
Chauhan, D., Hideshima, T., Mitsiades, C., Richardson, P., & Anderson, K. C. (2005). Proteasome inhibitor therapy in multiple myeloma. Molecular Cancer Therapeutics, 4(4), 686–692.
Kane, R. C., Dagher, R., Farrell, A., Ko, C.-W., Sridhara, R., Justice, R., et al. (2007). Bortezomib for the treatment of mantle cell lymphoma. Clinical Cancer Research, 13(18 Pt 1), 5291–5294.
Sartor, O. (2004). Overview of samarium Sm 153 lexidronam in the treatment of painful metastatic bone disease. Reviews in Urology, 6(Suppl 10), S3–S12.
Porter, A. T., McEwan, A. J., Powe, J. E., Reid, R., McGowan, D. G., Lukka, H., et al. (1993). Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int. J. Radiation Oncology Biol. Phys., 25, 805–813.
Baczyk, M., Czepczyński, R., Milecki, P., Pisarek, M., Oleksa, R., & Sowiński, J. (2007). 89Sr versus 153Sm-EDTMP: comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nuclear Medicine Communications, 28(4), 245–250.
Bauman, G., Charette, M., Reid, R., & Sathya, J. (2005). Radiopharmaceuticals for the palliation of painful bone metastases—a systematic review. Radiotherapy and Oncology, 75(3), 258. E1–258.E13.
Akerley, W., Butera, J., Wehbe, T., Noto, R., Stein, B., Safran, H., et al. (2002). A multiinstitutional, concurrent chemoradiation trial of strontium-89, estramustine, and vinblastine for hormone refractory prostate carcinoma involving bone. Cancer, 94(6), 1654–1660.
Tu, S. M., Millikan, R. E., Mengistu, B., Delpassand, E. S., Amato, R. J., Pagliaro, L. C., et al. (2001). Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet, 357, 336–341.
Harrison, M. R., Wong, T. Z., Armstrong, A. J., & George, D. J. (2013). Radium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Management and Research, 5, 1–14.
Rao, K. V. (2007). Lenalidomide in the treatment of multiple myeloma. American Journal of Health-System Pharmacy, 64(17), 1799–1807.
Murakami, H., Handa, H., Abe, M., Iida, S., Ishii, A., Ishikawa, T., et al. (2007). Low-dose thalidomide plus low-dose dexamethasone therapy in patients with refractory multiple myeloma. European Journal of Haematology, 79(3), 234–239.
Hicklin, D. J., & Ellis, L. M. (2005). Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of Clinical Oncology, 23(5), 1011–1027.
Chinot, O. L. (2012). Bevacizumab-based therapy in relapsed glioblastoma: rationale and clinical experience to date. Expert Review of Anticancer Therapy, 12(11), 1413–1427.
Flaherty, K. T. (2007). Sorafenib: delivering a targeted drug to the right targets. Expert Review of Anticancer Therapy, 7(5), 617–626.
Pantuck, A. J., Zomorodian, N., & Belldegrun, A. S. (2006). Phase I, open-label, single-center, multiple-dose, dose-escalation clinical study of SUO11248 (sunitinib) in subjects with high-risk prostate cancer who have elected to undergo radical prostatectomy. Clinical Cancer Research, 12, 4018–4026.
Drevs, J., Zirrgiebel, U., Schmidt-Gersbach, C. I. M., Mross, K., Medinger, M., Lee, L., et al. (2005). Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Annals of Oncology, 16(4), 558–565.
Smith, D. C., Smith, M. R., Sweeney, C., Elfiky, A. A., Logothetis, C., Corn, P. G., et al. (2013). Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. Journal of Clinical Oncology, 31(4), 412–419.
Eskens, F. A. L. M., Dumez, H., Hoekstra, R., Perschl, A., Brindley, C., Böttcher, S., et al. (2003). Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of cilengitide (EMD 121974), a novel inhibitor of the integrins αvβ3 and αvβ5 in patients with advanced solid tumours. European Journal of Cancer, 39(7), 917–926.
Mullamitha, S. A., Ton, N. C., Parker, G. J. M., Jackson, A., Julyan, P. J., Roberts, C., et al. (2007). Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clinical Cancer Research, 13(7), 2128–2135.
Tucker, G. C. (2006). Integrins: molecular targets in cancer therapy. Current Oncology Reports, 8(2), 96–103.
Gramoun, A., Shorey, S., Bashutski, J. D., Dixon, S. J., Sims, S. M., Heersche, J. N. M., et al. (2007). Effects of vitaxin, a novel therapeutic in trial for metastatic bone tumors, on osteoclast functions in vitro. Journal of Cellular Biochemistry, 102(2), 341–352.
Mulgrew, K., Kinneer, K., Yao, X.-T., Ward, B. K., Damschroder, M. M., Walsh, B., et al. (2006). Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Molecular Cancer Therapeutics, 5(12), 3122–3129.
Cheever, M. A., & Higano, C. S. (2011). PROVENGE (sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clinical Cancer Research, 17(11), 3520–3526.
Kantoff, P. W., Higano, C. S., Shore, N. D., Berger, E. R., Small, E. J., Penson, D. F., et al. (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine, 363(5), 411–422.
So-Rosillo, R., & Small, E. J. (2006). Sipuleucel-T (APC8015) for prostate cancer. Expert Review of Anticancer Therapy, 6(9), 1163–1167.
Park, J. W., Melisko, M. E., Esserman, L. J., Jones, L. A., Wollan, J. B., & Sims, R. (2007). Treatment with autologous antigen-presenting cells activated with the HER-2-based antigen lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. Journal of Clinical Oncology, 25(24), 3680–3687.
de Gruijl, T. D., van den Eertwegh, A. J. M., Pinedo, H. M., & Scheper, R. J. (2008). Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunology, Immunotherapy, 57(10), 1569–1577.
Kantoff, P. W., Schuetz, T. J., Blumenstein, B. A., Glode, L. M., Bilhartz, D. L., Wyand, M., et al. (2010). Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. Journal of Clinical Oncology, 28(7), 1099–1105.
Dayyani, F., Gallick, G. E., Logothetis, C. J., & Corn, P. G. (2011). Novel therapies for metastatic castrate-resistant prostate cancer. Journal of the National Cancer Institute, 103(22), 1665–1675.
Reck, M., Bondarenko, I., Luft, A., Serwatowski, P., Barlesi, F., Chacko, R., et al. (2013). Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Annals of Oncology, 24(1), 75–83.
Margolin, K., Ernstoff, M. S., Hamid, O., Lawrence, D., McDermott, D., Puzanov, I., et al. (2012). Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. The Lancet Oncology, 13(5), 459–465.
Sun, J., Schiffman, J., Raghunath, A., Ng Tang, D., Chen, H., & Sharma, P. (2008). Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immunity, 8, 9–15.
Fulton, A., Miller, F., Weise, A., & Wei, W.-Z. (2007). Prospects of controlling breast cancer metastasis by immune intervention. Breast Disease, 26, 115–127.
Melero, I., Grimaldi, A. M., Perez-Gracia, J. L., & Ascierto, P. A. (2013). Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clinical cancer research : an official journal of the American Association for Cancer Research, 19(5), 997–1008.
Fishelson, Z., Donin, N., Zell, S., Schultz, S., & Kirschfink, M. (2003). Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Molecular Immunology, 40, 109–123.
Pritchard-Jones, K., Spendlove, I., Wilton, C., Whelan, J., Weeden, S., Lewis, I., et al. (2005). Immune responses to the 105 AD7 human anti-idiotypic vaccine after intensive chemotherapy, for osteosarcoma. British Journal of Cancer, 92(8), 1358–1365.
Liao, Y., Schaue, D., & McBride, W. (2007). Modification of the tumor microenvironment to enhance immunity. Frontiers in Bioscience, 12, 3576–3600.
Dirkx, A. E. M., Oude Egbrink, M. G. A., Wagstaff, J., & Griffioen, A. W. (2006). Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. Journal of Leukocyte Biology, 80(6), 1183–1196.
Lewis, C. E., & Pollard, J. W. (2006). Distinct role of macrophages in different tumor microenvironments. Cancer Research, 66(2), 605–612.
Bingle, L., Brown, N. J., & Lewis, C. E. (2002). The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. Journal of Pathology, 196, 254–265.
Sica, A., Schioppa, T., Mantovani, A., & Allavena, P. (2006). Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. European Journal of Cancer, 42(6), 717–727.
Mantovani, A., Schioppa, T., Porta, C., Allavena, P., & Sica, A. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Reviews, 25(3), 315–322.
Porta, C., Kumar, B. S., Larghi, P., Rubino, L., Mancino, A., & Sica, A. (2007). Tumor promotion by tumor-associated macrophages. Advances in Experimental Medicine and Biology, 604, 67–86.
Loberg, R. D., Ying, C., Craig, M., Yan, L., Snyder, L. A., & Pienta, K. J. (2007). CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia, 9(7), 556–562.
Bailey, C., Negus, R., Morris, A., Ziprin, P., Goldin, R., Allavena, P., et al. (2007). Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clinical & Experimental Metastasis, 24(2), 121–130.
Craig, M. J., & Loberg, R. D. (2006). CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Reviews, 25(4), 611–619.
Pienta, K. J., Machiels, J.-P., Schrijvers D., Alekseev B., M. Shkolnik, & Crabb, S. J., et al. (2013). Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs, 31(3), 760–768.
Sandhu, S. K., Papadopoulos, K., Fong, P. C., Patnaik, A., Messiou, C., Olmos, D., et al. (2013). A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemotherapy and Pharmacology, 71(4), 1041–1050.
Baay, M., Brouwer, A., Pauwels, P., Peeters, M., & Lardon, F. (2011). Tumor Cells and Tumor-Associated Macrophages: Secreted Proteins as Potential Targets for Therapy. Clinical & Developmental Immunology, p. 565187.
Petit, I., Szyper-Kravitz, M., Nagler, A., Lahav, M., Peled, A., Habler, L., et al. (2002). G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology, 3(7), 687–694.
Shiozawa, Y., Pedersen, E. A., Havens, A. M., Jung, Y., Mishra, A., Joseph, J., et al. (2011). Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. Journal of Clinical Investigation, 121(4), 1298–1312.
Smith, M. C. P., Luker, K. E., Garbow, J. R., Prior, J. L., Jackson, E., Piwnica-Worms, D., et al. (2004). CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Research, 64(23), 8604–8612.
Meads, M. B., Hazlehurst, L. A., & Dalton, W. S. (2008). The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clinical Cancer Research, 14(9), 2519–2526.
Vincent, T., & Mechti, N. (2005). Extracellular matrix in bone marrow can mediate drug resistance in myeloma. Leukemia & Lymphoma, 46(6), 803–811.
Camacho, D. F., & Pienta, K. J. (2012). Disrupting the networks of cancer. Clinical Cancer Research, 18(10), 2801–2808.
Chen, K. W., & Pienta, K. J. (2011). Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theoretical Biology and Medical Modeling, 8(36), 1–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camacho, D.F., Pienta, K.J. A multi-targeted approach to treating bone metastases. Cancer Metastasis Rev 33, 545–553 (2014). https://doi.org/10.1007/s10555-013-9476-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9476-y