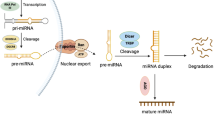
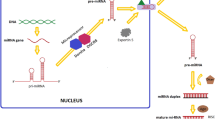
Abstract
Metastases are the major cause of cancer-related deaths, but the mechanisms of the metastatic process remain poorly understood. In recent years, the involvement of microRNAs (miRNAs) in cancer has become apparent, and the objective of this study was to identify miRNAs associated with breast cancer progression. Global miRNA expression profiling was performed on 47 tumor samples from 14 patients with paired samples from primary breast tumors and corresponding lymph node and distant metastases using LNA-enhanced miRNA microarrays. The identified miRNA expression alterations were validated by real-time PCR, and tissue distribution of the miRNAs was visualized by in situ hybridization. The patients, in which the miRNA profile of the primary tumor and corresponding distant metastasis clustered in the unsupervised cluster analysis, showed significantly shorter intervals between the diagnosis of the primary tumor and distant metastasis (median 1.6 years) compared to those that did not cluster (median 11.3 years) (p < 0.003). Fifteen miRNAs were identified that were significantly differentially expressed between primary tumors and corresponding distant metastases, including miR-9, miR-219-5p and four of the five members of the miR-200 family involved in epithelial-mesenchymal transition. Tumor expression of miR-9 and miR-200b were confirmed using in situ hybridization, which also verified higher expression of these miRNAs in the distant metastases versus corresponding primary tumors. Our results demonstrate alterations in miRNA expression at different stages of disease progression in breast cancer, and suggest a direct involvement of the miR-200 family and miR-9 in the metastatic process.



Similar content being viewed by others
References
(IARC) IAfRoC (2010) Globocan 2008. http://globocaniarcfr/factsheets/populations/factsheetasp?uno=900#KEY
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Tsuchiya S, Okuno Y, Tsujimoto G (2006) MicroRNA: biogenetic and functional mechanisms and involvements in cell differentiation and cancer. J Pharmacol Sci 101(4):267–270
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15–20
Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S et al (2005) MicroRNA gene expression deregulation in human breast cancer. Cancer Res 65(16):7065–7070
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D et al (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838
Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M et al (2008) MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 26(4):462–469
Rosenwald S, Gilad S, Benjamin S, Lebanony D, Dromi N, Faerman A et al (2010) Validation of a microRNA-based qRT-PCR test for accurate identification of tumor tissue origin. Mod Pathol 23(6):814–823
Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC et al (2009) A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137(6):1032–1046
Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD et al (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451(7175):147–152
Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY (2008) MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res 18(3):350–359
Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S et al (2008) The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 10(2):202–210
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic Acids Res 36(Database issue):D154–D158
Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39(Database issue):D152–D157
Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22(7):894–907
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G et al (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10(5):593–601
Birchmeier C, Birchmeier W, Brand-Saberi B (1996) Epithelial-mesenchymal transitions in cancer progression. Acta Anat 156(3):217–226
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564
Korpal M, Lee ES, Hu G, Kang Y (2008) The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283(22):14910–14914
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED et al (2007) Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol 213(2):374–383
Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D et al (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12(3):247–256
Zhu L, Chen H, Zhou D, Li D, Bai R, Zheng S, et al. (2011) MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol
Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M et al (2008) Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer 7:35
Hu X, Schwarz JK, Lewis JS Jr, Huettner PC, Rader JS, Deasy JO et al (2010) A microRNA expression signature for cervical cancer prognosis. Cancer Res 70(4):1441–1448
Tan HX, Wang Q, Chen LZ, Huang XH, Chen JS, Fu XH et al (2010) MicroRNA-9 reduces cell invasion and E-cadherin secretion in SK-Hep-1 cell. Med Oncol 27(3):654–660
Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M et al (2009) MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kappaB1. FEBS J 276(19):5537–5546
Wan HY, Guo LM, Liu T, Liu M, Li X, Tang H (2010) Regulation of the transcription factor NF-kappaB1 by microRNA-9 in human gastric adenocarcinoma. Mol Cancer 9:16
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist 57(1):289–300
Schopman NC, Heynen S, Haasnoot J, Berkhout B (2010) A miRNA-tRNA mix-up: tRNA origin of proposed miRNA. RNA Biol 7(5):573–576
Korpal M, Kang Y (2008) The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol 5(3):115–119
Fidler IJ, Kripke ML (1977) Metastasis results from preexisting variant cells within a malignant tumor. Science 197(4306):893–895
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871):530–536
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009
Kowalski PJ, Rubin MA, Kleer CG (2003) E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res 5(6):R217–R222
Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE et al (2000) Aberrant methylation of the estrogen receptor and E-cadherin 5′ CpG islands increases with malignant progression in human breast cancer. Cancer Res 60(16):4346–4348
Younis LK, El Sakka H, Haque I (2007) The prognostic value of E-cadherin expression in breast cancer. Int J Health Sci 1(1):43–51
Hwang HW, Wentzel EA, Mendell JT (2007) A hexanucleotide element directs microRNA nuclear import. Science 315(5808):97–100
Jeffries CD, Fried HM, Perkins DO (2011) Nuclear and cytoplasmic localization of neural stem cell microRNAs. RNA 17(4):675–686
Nass D, Rosenwald S, Meiri E, Gilad S, Tabibian-Keissar H, Schlosberg A et al (2009) MiR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol 19(3):375–383
Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V (2004) Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 5(3):R13
Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9(10):1274–1281
Lukiw WJ (2007) Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 18(3):297–300
Acknowledgments
We thank Lisbeth Mortensen and Ole Nielsen for excellent technical assistance with the immunohistochemical analysis and M. K. Occhipinti-Bender for editorial assistance. This study was supported in part by the Danish Cancer Society, the Danish Cancer Research Foundation, A Race Against Breast Cancer, Sino-Danish Breast Cancer Research Centre, NanoCAN Lundbeck Center of Excellence, and Danish Center for Translational Breast Cancer research (DCTB).
Ethical Standards
This manuscript complies with the currents laws in Denmark.
Conflict of interest
BSN is employee at Bioneer A/S. RS and TL are former employees of Exiqon A/S. All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gravgaard, K.H., Lyng, M.B., Laenkholm, AV. et al. The miRNA-200 family and miRNA-9 exhibit differential expression in primary versus corresponding metastatic tissue in breast cancer. Breast Cancer Res Treat 134, 207–217 (2012). https://doi.org/10.1007/s10549-012-1969-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-1969-9