Abstract
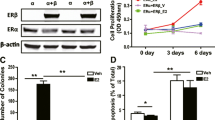
A large proportion of breast cancers expresses the estrogen receptor alpha (ERα) and are dependent on estrogens for their proliferation and survival. The tumor suppressor TP53 encodes the p53 protein, an important mediator of the anti-proliferative and apoptotic effects of several treatments used for breast cancer. A significant proportions of breast tumors (20–30%) carry mutations in TP53 gene and these mutations are associated with poor survival and poor response to several types of chemotherapeutic treatments. While there is mounting evidence for functional interactions between p53 and ERα pathways in breast and other tissues, the impact of these interactions on response to chemotherapy and anti-hormone treatments remain largely unknown. Here, using estrogen-dependent breast cancer cell lines with different p53 status, we show that estrogens, through ERα, influence p53 protein levels and activities. Estrogen deprivation reduced, while estradiol increased p53 levels, in a time and dose-dependent manner. Both wild-type and endogenously expressed mutant p53 proteins were affected. This reduction in p53 protein levels resulted in reduced p53-dependent responses induced by DNA damage in p53 wild-type cells, lowering the capacity of doxorubicine to induce apoptosis. The p53 response appeared to be quantitatively but not qualitatively affected. These results suggest that ERα activity is required for a strong p53 response in estrogen-dependent breast cancer cells. These results are in line with previous observations that we made in a clinical series, where a larger effect of TP53 mutation status was found for patient survival in cases with progesterone receptor positive status, a marker of a functional ERα pathway. It would thus be important to further characterize the influence of ERα pathway on the predictive value of TP53 mutation status in specifically designed clinical trials, as it may open perspectives for improving breast cancer treatment.



Similar content being viewed by others
References
Levine AJ, Momand J, Finlay CA (1991) The p53 tumour suppressor gene. Nature 351:453–456
Hainaut P, Hollstein M (2000) p53 and human cancer: the first ten thousand mutations. Adv Cancer Res 77:81–137
Olivier M, Hainaut P, Borresen-Dale A (2005) Prognostic and predictive value of TP53 mutations in human cancer. In: Hainaut P, Wiman K (eds) 25 years of p53 research. Springer, pp 321–338
Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931
Cheskis BJ, Greger JG, Nagpal S, Freedman LP (2007) Signaling by estrogens. J Cell Physiol 213:610–617
Santen RJ, Fan P, Zhang Z, Bao Y, Song RX, Yue W (2009) Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids 74:586–594
Duong V, Boulle N, Daujat S, Chauvet J, Bonnet S, Neel H, Cavailles V (2007) Differential regulation of estrogen receptor alpha turnover and transactivation by Mdm2 and stress-inducing agents. Cancer Res 67:5513–5521
Liu G, Schwartz JA, Brooks SC (2000) Estrogen receptor protects p53 from deactivation by human double minute-2. Cancer Res 60:1810–1814
Hurd C, Dinda S, Khattree N, Moudgil VK (1999) Estrogen-dependent and independent activation of the P1 promoter of the p53 gene in transiently transfected breast cancer cells. Oncogene 18:1067–1072
Moudgil VK, Dinda S, Khattree N, Jhanwar S, Alban P, Hurd C (2001) Hormonal regulation of tumor suppressor proteins in breast cancer cells. J Steroid Biochem Mol Biol 76:105–117
Shirley SH, Rundhaug JE, Tian J, Cullinan-Ammann N, Lambertz I, Conti CJ, Fuchs-Young R (2009) Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res 69:3405–3414
Angeloni SV, Martin MB, Garcia-Morales P, Castro-Galache MD, Ferragut JA, Saceda M (2004) Regulation of estrogen receptor-alpha expression by the tumor suppressor gene p53 in MCF-7 cells. J Endocrinol 180:497–504
Liu G, Schwartz JA, Brooks SC (1999) p53 down-regulates ER-responsive genes by interfering with the binding of ER to ERE. Biochem Biophys Res Commun 264:359–364
Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, Hager JA, Beliakoff J, Whitesell LJ, Bowden GT, Romagnolo DF (2005) An estrogen receptor-alpha/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: repressive effects of p53 on BRCA-1 transcription. Neoplasia 7:873–882
Jin W, Chen Y, Di GH, Miron P, Hou YF, Gao H, Shao ZM (2008) Estrogen Receptor (ER) beta or p53 attenuates ER{alpha}-mediated transcriptional activation on the BRCA2 promoter. J Biol Chem 283:29671–29680
Liu W, Konduri SD, Bansal S, Nayak BK, Rajasekaran SA, Karuppayil SM, Rajasekaran AK, Das GM (2006) Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem 281:9837–9840
Lewandowski SA, Thiery J, Jalil A, Leclercq G, Szczylik C, Chouaib S (2005) Opposite effects of estrogen receptors alpha and beta on MCF-7 sensitivity to the cytotoxic action of TNF and p53 activity. Oncogene 24:4789–4798
Molinari AM, Bontempo P, Schiavone EM, Tortora V, Verdicchio MA, Napolitano M, Nola E, Moncharmont B, Medici N, Nigro V, Armetta I, Abbondanza C, Puca GA (2000) Estradiol induces functional inactivation of p53 by intracellular redistribution. Cancer Res 60:2594–2597
Menendez D, Inga A, Snipe J, Krysiak O, Schonfelder G, Resnick MA (2007) A single-nucleotide polymorphism in a half-binding site creates p53 and estrogen receptor control of vascular endothelial growth factor receptor 1. Mol Cell Biol 27:2590–2600
Lowe SW (1995) Cancer therapy and p53. Curr Opin Oncol 7:547–553
Olivier M, Langerod A, Carrieri P, Bergh J, Klaar S, Eyfjord J, Theillet C, Rodriguez C, Lidereau R, Bieche I, Varley J, Bignon Y, Uhrhammer N, Winqvist R, Jukkola-Vuorinen A, Niederacher D, Kato S, Ishioka C, Hainaut P, Borresen-Dale AL (2006) The clinical value of somatic TP53 gene mutations in 1, 794 patients with breast cancer. Clin Cancer Res 12:1157–1167
Shaulian E, Zauberman A, Ginsberg D, Oren M (1992) Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol 12:5581–5592
Salvat C, Aquaviva C, Jariel-Encontre I, Ferrara P, Pariat M, Steff AM, Carillo S, Piechaczyk M (1999) Are there multiple proteolytic pathways contributing to c-Fos, c-Jun and p53 protein degradation in vivo? Mol Biol Rep 26:45–51
Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW (1999) Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862
Wijayaratne AL, McDonnell DP (2001) The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem 276:35684–35692
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC (2001) HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 3:973–982
Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387:299–303
Schiff R, Massarweh S, Shou J, Osborne CK (2003) Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 9:447S–454S
Becker KA, Lu S, Dickinson ES, Dunphy KA, Mathews L, Schneider SS, Jerry DJ (2005) Estrogen and progesterone regulate radiation-induced p53 activity in mammary epithelium through TGF-beta-dependent pathways. Oncogene 24:6345–6353
Dunphy KA, Blackburn AC, Yan H, O’Connell LR, Jerry DJ (2008) Estrogen and progesterone induce persistent increases in p53-dependent apoptosis and suppress mammary tumors in BALB/c-Trp53 ± mice. Breast Cancer Res 10:R43
Guillot C, Falette N, Courtois S, Voeltzel T, Garcia E, Ozturk M, Puisieux A (1996) Alteration of p53 damage response by tamoxifen treatment. Clin Cancer Res 2:1439–1444
Adhikari AS, Iwakuma T (2009) Mutant p53 gain of oncogenic function: in vivo evidence, mechanism of action and its clinical implications. Fukuoka Igaku Zasshi 100:217–228
Brosh R, Rotter V (2009) When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9:701–713
Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G (2008) The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev 22:1337–1344
Acknowledgments
This study has been supported by a grant from the Association for International Cancer Research. We thank Ke-Seay Smoth for her technical help.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernández-Cuesta, L., Anaganti, S., Hainaut, P. et al. Estrogen levels act as a rheostat on p53 levels and modulate p53-dependent responses in breast cancer cell lines. Breast Cancer Res Treat 125, 35–42 (2011). https://doi.org/10.1007/s10549-010-0819-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0819-x