Abstract
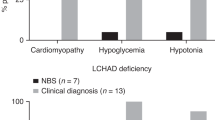
Experience with new-born screening (NBS) for disorders of fatty-acid oxidation (FAOD) is now becoming available from an increasing number of programs worldwide. The spectrum of FAOD differs widely between ethnic groups. Incidence calculations from reports from Australia, Germany, and the USA of a total of 5,256,999 newborns give a combined incidence of all FAOD of approximately 1:9,300. However, it appears to be much lower in Asians. Consequently, a significant prevalence and evidence for a clear benefit of NBS is proven for medium-chain acyl-CoA dehydrogenase deficiency (MCAD) only in countries with a high percentage of Caucasians, with very-long-chain acyl-CoA dehydrogenase deficiency (VLCAD) and long-chain 3-hydroxy acyl-CoA dehydrogenase deficiency (LCHAD) being additional candidates. The long-term benefit for many disorders has still to be evaluated and will require international collaboration, especially for the rarest disorders. Short-chain acyl-CoA dehydrogenase deficiency (SCAD) [as well as Systemic carnitine transporter deficiency (CTD) and dienoyl-CoA reductase deficiency (DE-RED)] are conditions of uncertain clinical significance, but most FAOD have a spectrum of clinical presentations (healthy–death). Confirmatory diagnostic procedures should be agreed upon to ensure international comparability of results and evidence-based modifications. The case of short-chain acyl-CoA dehydrogenase deficiency (SCAD) deficiency shows that even inclusion of conditions without a clearly known natural course may prove useful with respect to gain of knowledge and consecutive exclusion of a biochemical abnormality without clinical significance, although this line of argument implies the existence of structured follow-up programs and bears ethical controversies. As a final conclusion, the accumulated evidence suggests all FAOD should to be included into tandem mass spectrometry (MS/MS)-based NBS programs provided sufficient laboratory performance is guaranteed.
Similar content being viewed by others
Abbreviations
- NBS:
-
Newborn screening
- FAOD:
-
Fatty-acid oxidation disorders
- MCAD:
-
Medium-chain acyl-CoA dehydrogenase deficiency
- VLCAD:
-
Very-long-chain acyl-CoA dehydrogenase deficiency
- LCHAD/mTFP:
-
Long-chain 3-hydroxy acyl-CoA dehydrogenase deficiency/mitochondrial trifunctional protein deficiency
- CTD:
-
Systemic carnitine transporter deficiency
- SCAD:
-
Short-chain acyl-CoA dehydrogenase deficiency
- CPT I:
-
Carnitine palmitoyltransferase 1 deficiency
- CPT II:
-
Carnitine palmitoyltransferase-2 deficiency
- CACT:
-
Carnitine acylcarnitine translocase deficiency
- MAD/GA II:
-
Multiple acyl-CoA dehydrogenase deficiency/glutaric aciduria type II (synonym)
- MCKAT:
-
Medium-chain ketothiolase
- M/SCHAD:
-
Medium-/short-chain 3-OH-acyl-CoA dehydrogenase deficiency
- DE-RED:
-
Dienoyl-CoA reductase deficiency
- MS/MS:
-
Tandem mass spectrometry
References
Boneh A, Andresen BS, Gregersen N et al (2006) VLCAD deficiency: pitfalls in newborn screening and confirmation of diagnosis by mutation analysis. Mol Genet Metab 88:166–170
Huang HP, Chu KL, Chien YH, Wie ML, Wu ST, Wang SF, Hwu WL (2006) Tandem mass neonatal screening in Taiwan—report from one center. Formos Med Assoc 105:882–886
Maier EM, Pongratz J, Muntau AC et al (2009) Validation of MCADD newborn screening. Clin Genet 76:179–187
Nichols MJ, Saavedra-Matiz CA, Pass KA, Caggana M (2008) Novel mutations causing medium chain acyl-CoA dehydrogenase deficiency: under-representation of the common c.985 A > G mutation in the New York state population. Am J Med Genet A 146A:610–619
Pandor A, Eastham J, Berverley C et al (2004) Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review. Health Technol Assess 8:1–152
Rinaldo P, Zafari S, Tortorelli S, Matern D (2006) Making the case for objective performance metrics in newborn screening by tandem mass spectrometry. Ment Retard Dev Disabil Res Rev 12:255–261
Schulze A, Lindner M, Kohlmüller D et al (2003) Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics 111:1399–1406
van der Hilst CS, Derks TGJ, Reijngoud DJ et al (2007) Cost-effectiveness of neonatal screening for medium chain acyl-CoA dehydrogenase deficiency: the homogenous population of the Netherlands. The J Pediatr 151:115–120
van der Kamp HJ, Wit JM (2004) Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol 151(Suppl 3):U71–U75
van Maldegem BT, Duran M, Wanders RJA (2006) Clinical, biochemical, and genetic heterogeneity in short-chain acyl-coenzyme a dehydrogenase deficiency. JAMA 296:943–952
Waisbren SE, Levy HL, Noble M, Matern D, Gregersen N, Pasley K, Marsden D (2008) Short-chain acyl-CoA dehydrogenase (SCAD) deficiency: an examination of the medical and neurodevelopmental characteristics of 14 cases identified through newborn screening or clinical symptoms. Mol Genet Metab 95:39–45
Watson MS, Mann MY, Lloyd-Puryear MA et al (2006) Newborn screening: towards a uniform screening panel. Genet Med 8:s1–s11
Wilcken B, Haas M, Joy P, Wiley V, Bowling F, Carpenter K, Christodoulou J, Cowley D, Ellaway C, Fletcher J, Kirk EP, Lewis B, McGill J, Peters H, Pitt J, Ranieri E, Yaplito-Lee J, Boneh A (2009) Expanded newborn screening: outcome in screened and unscreened patients at age 6 years. Pediatr 124:e241–e248
Wilson JM, Jungner G (eds) (1968) Principles and practice of screening for disease. World Health Organization. Available at http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. Accessed 21 Mar 2010
Zytkovic T, Fitzgerald EF, Marsden D et al (2001) Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England newborn screening program. Clin Chem 47:1945–1955
Acknowledgements
We thank all colleagues who participated and contributed to the discussion, especially Dr. Olaf Bodamer, Austria, Dr. Andreas Schulze, Canada, Dr. Ute Spiekerkötter, Germany, and Dr. Bridget Wilcken, Australia. The work of Dr. Lindner and Dr. Hoffmann is generously sponsored by the Dietmar-Hopp-Stiftung, Walldorf GmbH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Verena Peters
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Lindner, M., Hoffmann, G.F. & Matern, D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis 33, 521–526 (2010). https://doi.org/10.1007/s10545-010-9076-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9076-8