Abstract
The nature of linkages between soil C and N cycling is important in the context of terrestrial ecosystem responses to global environmental change. Extracellular enzymes produced by soil microorganisms drive organic matter decomposition, and are considered sensitive indicators of soil responses to environmental variation. We investigated the response of eight hydrolytic soil enzymes (four peptidases and four glycosidases) to experimental warming in a long-term climate manipulation experiment in a sub-arctic peat bog, to determine to what extent the response of these two functional groups are similar. We found no significant effect of experimental spring and summer warming and/or winter snow addition on either the potential activity or the temperature sensitivity (of Vmax) of any of the enzymes. However, strong and contrasting seasonal patterns in both variables were observed. All of the peptidases, as well as alpha-glucosidase, had lower potential activity at the end of summer (August) compared to the beginning (June). Conversely, beta-glucosidase had significantly higher potential activity in August. Peptidases had consistently higher temperature sensitivities in June compared to August, while all four glycosidases showed the opposite pattern. Our results suggest that warming effects on soil enzymes are small compared to seasonal differences, which are most likely mediated by the seasonality of substrate supply and microbial nutrient demand. Furthermore the contrasting seasonal patterns for glycosidases and peptidases suggest that enzyme-based models of soil processes need to allow for potential divergence between the production and activity of these two enzyme functional groups.
Similar content being viewed by others
Introduction
The key processes in terrestrial biogeochemistry—primary production, decomposition, and nutrient cycling—are largely controlled by the interaction of cycles of biologically important elements. These cycles are generally closely coupled due to basic constraints on the elemental composition of biological macromolecules leading to an emergent stoichiometry of processes from the molecular to ecosystem level (Sterner and Elser 2002). In many ecosystems, the interaction between C and N cycles is of particular importance. For example, available nitrogen often limits gross primary production (LeBauer and Treseder 2008), can alter patterns of C allocation in forests (Vicca et al. 2012), and may influence the rate of soil organic matter turnover and thus control soil carbon storage (Janssens et al. 2010; Knicker 2011). The current research effort to understand terrestrial carbon cycling in the context of climate change therefore requires understanding of the interactions between C and N cycles, as well as the response of these linkages to global changes such as climate warming (Finzi et al. 2011).
Decomposition of organic matter in soils is an important point of connection between terrestrial C and N cycles. The rate of breakdown of polymeric forms of C determines the magnitude of carbon storage and emissions of greenhouse gases, and has feedback effects on primary production by making organic and mineral N available for re-uptake by plants. At large scales decomposition is controlled by environmental conditions and, in some contexts, the chemical composition of the organic matter inputs (Aerts 1997; but see Schmidt et al. 2011). However, at the molecular scale the proximate drivers are exoenzymes produced by soil microbes, which catalyse the hydrolytic and oxidative reactions that breakdown polymeric organic substances (Nannipieri et al. 2002). Bacteria and fungi produce a wide range of enzymes, each specialized for the breakdown of specific classes of molecules e.g. alpha-glucosidase (starch), cellulase (cellulose), phenol oxidases (complex polymeric substances such as lignin) and proteases and peptidases (polypeptides) (Skujinš 1976). The production of these enzymes is regulated by both the availability of the substrate, and the microbial demand for the corresponding product (Allison and Vitousek 2005; Allison et al. 2011). Moreover, the enzymes themselves are N-rich and energetically costly to produce. The relative levels of production and activity of different groups of soil extracellular enzymes therefore reflects the physiological constraints and economic trade-offs within the microbial community (Schimel et al. 2007; Allison et al. 2010).
Recent developments in decomposition modelling have incorporated the dynamics and physiology of microbial biomass and the production and activity of extracellular enzymes (e.g. Schimel and Weintraub 2003; Moorhead and Sinsabaugh 2006; Allison et al. 2010). These models reveal the potential for subtle interactions between environmental conditions, substrate supply and decomposition rates, and have shed light on previously puzzling phenomena such as the observation that N addition can suppress decomposition rates (Craine et al. 2007). Moreover, there is an ongoing discussion about how best to use enzymes to improve the realism of large-scale earth system models (Todd-Brown et al. 2012). However, a common assumption of enzyme-based models is that the dynamics of C- and N-acquiring enzymes can be modelled together, that is, that they show similar responses to environmental variability in terms of levels of enzyme production. Whether this assumption is valid, particularly in terms of the similarity of responses to climate change phenomena, is an important question with implications for the realism of model predictions of soil organic matter decomposition.
There have been several recent studies examining the effects of experimental warming on soil enzyme activities (reviewed in Henry 2012). These studies usually measure the potential activities (pool sizes) of various enzymes, but shifts in enzyme kinetics (temperature sensitivity of maximum reaction rate and/or substrate affinity) may also play an important role in in situ soil responses to warming (Wallenstein et al. 2011). It has been shown that temperature sensitivity (Q10) of maximum enzyme reaction rates (Vmax) can vary with season and/or soil type (Trasar-Cepeda et al. 2007; Wallenstein et al. 2009), but how this parameter is affected by long term soil warming is unknown. Furthermore, studies of warming effects on soil enzymes tend to examine each enzyme separately, whereas an integrative approach that explicitly compares the dynamics of a range enzymes involved in both the C and N cycles may help to elucidate the extent to which they are subject to the same environmental controls.
In this study, we investigated the response of a range of hydrolytic soil enzymes to experimental climate manipulation in a long-term experiment in northern Sweden. We measured four enzymes each from two functional groups. We measured glycosidases as indicators of soil C cycling. These enzymes catalyze hydrolytic degradation of polymeric carbohydrates such as cellulose and starch into simpler sugars. To investigate organic N cycling we assayed four amino-peptidases-enzymes which catalyse the hydrolysis of peptide bonds in polypeptides. It is important to note that peptidase activity releases assimilable C as well as N, which complicates its interpretation as an indicator of N-cycle dynamics (Sinsabaugh and Foreman 2003). For the purposes of our interpretation we make the assumption that peptidase activity is related to N supply and/or microbial demand in a meaningful sense (Allison and Vitousek 2005; Sinsabaugh et al. 2008), but we cannot strictly rule out a simultaneous relationship with C dynamics.
Our study particularly focused on testing whether or not enzymes from the two functional groups responded similarly to the climate treatments. Furthermore, we used samples from the beginning and end of the summer growing season, as both potential activity and temperature sensitivity can show significant seasonal variation (Bonnett et al. 2006; Wallenstein et al. 2009; Bell et al. 2010), and therefore any patterns of response to warming may vary over time. Our experimental site is located in a sub-arctic peatland, a biome of special significance in the global carbon cycle due to its status as a long term carbon sink (Limpens et al. 2008) in a region that is particularly vulnerable to climate warming (ACIA 2004). The links between C and N cycles in this system are also important: N availability strongly limits C cycle processes (Rosswall and Granhall 1980), and components of both cycles have been shown to respond strongly to climate warming (Dorrepaal et al. 2009; Weedon et al. 2012).
Our study addresses the following questions:
-
1)
How do the potential activities and temperature sensitivities of soil glycosidases and peptidases respond to experimental climate manipulation?
-
2)
Do the enzyme activities and temperature sensitivities vary between the beginning and end of the growing season?
-
3)
Do soil glycosidases and peptidases show similar responses (i.e. same direction) to experimental climate manipulation and/or across the season?
Materials and methods
Study site and sampling
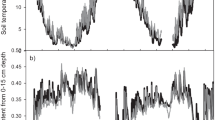
Soil samples for enzyme analysis were collected from a long-term climate manipulation experiment established on a blanket bog about 800 m from the Abisko Scientific Research Station in Abisko, sub-arctic Sweden (68°21′N, 18°49′E, alt. 340 m) (Dorrepaal et al. 2004). The gently sloping site is dominated by Sphagnum sp. mosses (predominantly Sphagnum fuscum), with a sparse cover of dwarf shrubs Empetrum hermaphroditum, Rubus chaemomorus, Betula nana and Vaccinium uliginosum. The climate is continental sub-arctic with mean annual rainfall of 352 mm and mean monthly temperatures in January and July of −9.7 and 12.3 °C respectively (meteorological data 1999–2008, Abisko Scientific Research Station). Since 2000 a climate manipulation experiment has been maintained to investigate the effects of summer and spring warming and winter snow accumulation on a range of soil and vegetation processes (Dorrepaal et al. 2004; Aerts et al. 2007; Dorrepaal et al. 2009; Keuper et al. 2011; Aerts et al. 2012). The experiment consists of factorial combinations of summer treatments (ambient or warming), and winter/spring treatments (ambient and snow addition + spring warming) randomly assigned to plots in 5 contiguous blocks parallel to the bog slope (i.e. 5 plots per 4 treatments = 20 plots total). Treatments are applied using open top chambers (OTCs): hexagonal, transparent polycarbonate structures 50 cm high, and with diameter 1.6–1.8 m at the top and 2.2–2.5 m at the base. For summer warning plots the OTCs are in place every year from the beginning of June until the end of September. For snow-addition + spring warming plots the OTCs are also in place for the remainder of the year (Dorrepaal et al. 2004). The OTCs increase average daily mean air temperature by 0.3–1.0 °C in spring (April–June) and by 0.2–0.9 °C in summer (June–October). Effects on soil temperature vary with depth and from plot to plot: but typically passive air warming also increases average soil temperature at 10 cm depth by 0.6–1.0 °C. During winter, there is a passive accumulation of snow leading to approximately a doubling of the snow layer thickness and an increase of winter average soil temperature of 0.5–2.2 °C (Dorrepaal et al. 2004; Dorrepaal et al. 2009). In spring the melting residue of this increased snow cover can also locally counteract the spring warming effect. Soil temperature in a subset of plots at 5, 10 and 20 cm depth is logged year-round at two-hourly intervals using methodology detailed in Dorrepaal et al. (2004).
Soil for enzyme analyses was sampled on June 4, 2009 and August 16, 2009. These dates were chosen to roughly coincide with the beginning of the vegetation growing season (June) and the latter part of the growing season, prior to vascular plant senescence (August). Soil cores of 5 cm diameter were taken to 15 cm depth near the centre of each plot. At this depth the soil is primarily partially decomposed Sphagnum material (i.e. Oi horizon) with organic matter content >95 % and % C approximately 45 % (±0.64 standard error; E. Dorrepaal, pers.comm). Within 12 h of sampling, samples were mixed and all coarse roots and live moss removed. Subsamples were taken for immediate measurement of soil water content. The remaining material was then immediately stored at −20 °C and transported to Amsterdam for further analyses. Although performing assays on freshly sampled material is preferable, logistic constraints made this impractical, and there is evidence that freezing at −20 °C does not significantly alter measured enzyme activities relative to cool storage (DeForest 2009). It should be noted, however, that although relative treatment effects were most likely not influenced by storage, absolute values of potential enzyme activity (see “Results” section below) may deviate from those that would have been obtained from freshly sampled soil.
Enzyme assays
Potential enzyme activity assays were conducted as described in Weedon et al. (2012) using the protocol of Steinweg and McMahon (http://enzymes.nrel.colostate.edu/). Model substrates labeled with the fluorophores 7-amino-4-methylcoumarin (MUC) or methylumbelliferone (MUB) were used to quantify the relative pool sizes (i.e. activity under saturating substrate concentrations) of enzymes responsible for the hydrolysis of four peptide and four carbohydrate substrates. The specific model substrates used were, for peptidase activity: l-leucine-7-amido-4-MUC (henceforth, Leucine), l-alanine-7-amido-4-MUC (Alanine), l-lysine-alanine-7-amido-4-MUC (Lys-Ala), and l-alanine-alanine-phenylalanine-7-amido-4-MUC (AAP); and for glycosidase activity: 4-MUB-B-d-xylopyranoside (xylase), 4-MUB-β-glucopyranoside (beta), 4-MUB-α-glucopyranoside (alpha) and 4-MUB-β-cellobioside (cellulase) (all substrates supplied by Sigma-Aldrich). The protocol includes construction of seven-point calibration curves for each individual slurry preparation and thus provides better correction for non-linear fluorescence quenching dynamics than the usual single-point correction.
We followed the protocol cited above using slurries created by homogenizing 4 g fresh weight of soil in 90 mL 0.5 M sodium acetate buffer (pH 5). Incubations were conducted at two temperatures (4 °C for 22 h or 20 °C for 4 h) to allow calculation of the temperature sensitivity of the hydrolysis of each of the model substrates. Temperature sensitivity estimates can depend on the choice of temperature range (Chapman and Thurlow 1998). In this study temperatures were chosen to reflect the natural range of soil temperatures at the site during the study period (see Fig. 1). End-point fluorometric measurements were made on a Spectramax Gemini XS microplate fluorometer (Molecular Devices, Sunnyvale, USA) with excitation wavelength of 365 nm and emission detection at 450 nm. All measurements were converted to nanomoles per gram dry weight per hour for statistical analysis.
Temperature sensitivities for enzyme activity were calculated for each sample × enzyme combination using the formula:
where Q10 is the factor increase in enzyme activity with a 10 degrees increase in temperature, and A20 and A4 are the measured enzyme potential activities at 20 and 4 °C, respectively.
Statistical analysis
To test for the (possibly interactive) effects of climate manipulation treatments and sampling time on potential enzyme activities and temperature sensitivities, we fitted mixed-effects models for each enzyme separately (and for enzyme activities, separately for measurements at 4 and 20 °C). Sampling month, summer treatment and winter/spring treatment (and all two- and three-way interactions thereof) were included as fixed effects and plot ID modelled as a random plot-level intercept to account for non-independence of repeated samples taken from the same plot. The parameter estimates from these analyses suggested contrasting patterns of seasonal responses across the different enzymes, which we subsequently tested in a larger, four-way factorial mixed model combining all measurements (centred and scaled per enzyme, due to the large range in absolute values, and to focus on patterns in relative responses within each enzyme to treatment and sampling time). In this model we treated enzyme as a fixed factor, and all other factors as described for the three-way models above.
Results
The summer warming treatment led to soil warming over the period of the sampling (Fig. 1). The daily average temperature at 5 cm depth in summer warmed plots was, on average, 1.1 °C higher than in ambient plots between May 6 (1 month before first sampling) and August 16 (second sampling). Over the same period, daily maxima were on average 2.3 °C higher in warmed plots, with no difference in daily minima (n = 8 monitored plots for each treatment). The effects of the “spring warming + snow addition” treatment on soil temperatures in the 1 month preceding sampling was not statistically significant from ambient plots (ANOVA, P > 0.05)—due to a high variability in soil temperatures between these plots (data not shown), most likely due to patchily distributed residual snow.
Soil moisture content was not significantly affected by climate treatments but showed a significant (repeated measures ANOVA, P < 0.05) decline from June to August. The decline was, however, small in magnitude: from 88.0 ± 0.3 % water by weight (mean ± SE) in June, to 86.9 ± 0.5 % in August.
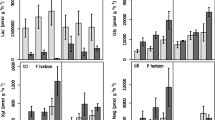
There was no significant effect of climate manipulation treatment on either the potential activity at 4 or 20 °C, or on temperature sensitivity of any of the eight measured enzymes (Tables 1 and 2). There was, however, a significant effect of sampling time on potential activity at 20 °C and this effect was enzyme dependent (Table 2, enzyme × month interaction P < 0.05). When tested individually, 6 out of the 8 enzymes, showed this seasonal effect. All peptidases had lower potential activity in August compared to June. Of the four glycosidases, only alpha-glucosidase had a lower activity in August (Fig. 2). Beta-glucosidase showed the opposite pattern, having significantly higher potential activity in August, and cellulase and xylase potential activity did not significantly change between the sampling events (Fig. 2). Almost identical patterns were seen in the measurements made at 4 °C, with the exception that lysine-alanine-peptidase showed no significant seasonal shift, while cellulase and xylase potential activity also decreased in potential activity between June and August (data not shown).
Mean (±95 % confidence interval) seasonal shifts (June to August) in potential enzyme activity measured at 20 °C. Negative values indicate reduction in potential activity over the growing season. Data for each enzyme are centred and scaled by standard deviation, to allow comparison across enzyme types, unscaled values are given in Table 1
Similarly, apparent temperature sensitivity differed on an enzyme specific-basis between the two sampling moments (Table 2, enzyme × month interaction P < 0.05). All four peptidases showed a decrease in temperature sensitivity from June to August, whereas the glycosidases showed the opposite pattern (Fig. 3). The mean values of Q10 for the glycosidases were 1.8 (±0.2, standard error of mean) in June and 2.9 (±0.3) in August, whereas for the peptidases the values were 2.6 (±0.1) in June and 1.9 (±0.1) in August.
Discussion
The experimental climate manipulations imposed in our study had no effect on either the potential activities or temperature sensitivities of any of the 8 enzymes we assayed, a result that contrasts with the clear effects of the same treatments on soil respiration, nitrogen cycling and vegetation growth (Dorrepaal et al. 2009; Keuper et al. 2011; Weedon et al. 2012). On the other hand, we observed striking seasonal patterns in both potential activity and temperature sensitivity of almost all of the enzymes. These results have implications for our understanding of seasonal nutrient dynamics in this system, and raise issues about the incorporation of soil enzyme dynamics into models of soil processes and their responses to global change.
Lack of warming effect on soil enzymes
Observational and experimental field studies in a range of systems have failed to find a straightforward relationship between temperature and potential enzyme activity (Bonnett et al. 2006; Bell et al. 2010; Bell and Henry 2011; Henry 2012). In our study, a similar result is perhaps not surprising given the modest amount of experimental warming (+1 °C). However, temperatures increases of similar magnitude translate to increased rates of soil respiration and nitrogen turnover in a range of systems (Rustad et al. 2001). Moreover, in our experimental system, both of these fluxes have been observed to increase dramatically (50–100 % increase) under the same modest warming regime (Dorrepaal et al. 2009; Weedon et al. 2012). The corresponding lack of detectable changes in soil enzyme pools imply that these effects are not caused by changes in the pool sizes of the enzymes involved in the transformations. One explanation for this discrepancy is that enzyme activity assays measure potential activity under non-limiting levels of substrate (Allison et al. 2007). It is therefore possible that warming effects on C and N fluxes could be related to changes in the supply of substrate, as this can lead to large differences in in situ turnover rates of C and N without differences in the measured potential activity of the enzymes involved in the fluxes (Weintraub and Schimel 2005). Another, nonexclusive, explanation is that other enzyme pathways, not measured in this study, are more closely linked to in situ flux rates than the glycosidases and amino-peptidases we measured in this study. For nitrogen cycling, chitinases and endo-protease enzymes may also be important (Caldwell 2005). In the carbon cycle, oxidative enzymes may be the key control on flux rates (Freeman et al. 2001). More generally, the extent to which we can link enzyme measurements to variation in environmental conditions and ecosystem processes of interest remains a key challenge in soil enzymology (Allison et al. 2007; Weedon et al. 2011; Henry 2012).
Seasonality of enzyme pool sizes and temperature sensitivities
Despite the lack of effects of climate treatments, the strong seasonality in the relative sizes of enzyme pools suggests that they are subject to some degree of regulation. Enzyme pools at a given moment in time are the net result of processes of production, degradation and immobilization. Thus, we cannot definitively assert that this regulation is actively exerted by the microbial community, or even if we could, whether this regulation occurs at the level of changes in the composition of the enzyme-producing community (Allison and Martiny 2008), or through shifts in the production of enzymes within an otherwise stable community. However, the changes in temperature sensitivity can be interpreted as a shift in the isoenzyme composition of the enzyme pools (Wallenstein et al. 2011), where isoenzymes are groups of enzymes with the same catalytic function but different structure. If this is the case it could be considered as evidence in favour of microbe-level control of enzyme pools. For the purposes of the following discussion, we assume that the patterns we have observed are adaptive—and can be interpreted in terms of the nutritional status of the soil microbial community.
Control of extracellular enzyme production by soil microbes can be understood in terms of ecological-economic constraints (Allison et al. 2011). Microbes should allocate resources to specific enzymes that allow them to obtain resources which are limiting growth and reproduction. At the same time, substrate-induction of enzyme production could also be important, as there is no energetic benefit in producing enzymes for which the corresponding substrate is not present in the surrounding environment (Allison and Vitousek 2005; German et al. 2011). In this framework, if we assume peptidase production is driven primarily by N demand, the higher potential activities of these enzymes in June relative to August may reflect a greater relative demand for nitrogen earlier in the season. This is supported by the relatively lower concentrations of inorganic N in June (Weedon et al. 2012). This interpretation may also help explain the corresponding increase in beta-glucosidase activity across the growing season. The microbial biomass could be shifting from demand for nitrogen in June to demand for carbon/energy in August, with corresponding shifts in the relative production of peptidases and glucosidases (the implications of this temporal divergence of C and N dynamics for modeling are explored further below). However, the data for the other C cycle enzymes suggest a more complex picture. In terms of pool sizes, alpha-glucosidase shows a magnitude of relative decline comparable to that seen for the four peptidases. A possible explanation is that the production of this enzyme is controlled more by substrate supply than demand, as was demonstrated in experiments demonstrating a positive relationship between starch concentration and alpha-glucosidase activity (German et al. 2011). More generally, the contrasting patterns between beta-glucosidase and alpha-glucosidase potential activities may indicate a shift in allocation from enzymes degrading more labile C sources (starch) early in the season towards cellulose metabolism later in the season when labile C sources are scarcer.
It is important to bear in mind that the foregoing interpretation of seasonal dynamics is based on only two sampling occasions, a consequence of the need to limit destructive sampling in a long-term, multi-use experiment. The seasonal patterns we tentatively identify would need to be validated by more intensive temporal sampling, preferably in a wider variety of systems. Nevertheless, our interpretation broadly agrees with the findings of Weintraub and Schimel (2005), who concluded that an early season peak in protease activity in tundra soils was driven by increased microbial demand for N and speculated that this microbial N limitation was partly driven by a transient increase in the supply of labile C by plants. In general, seasonally frozen soils, such as those in (sub-) arctic and alpine environments are characterized by a strong seasonality in the availability, mobilization, and uptake of carbon and nitrogen (Falge et al. 2002). This is a consequence of rapid turnover of microbial biomass during spring-thaw (Schmidt et al. 2007), and the temporal dynamics of plant nutrient uptake and rhizodeposition during the relatively short plant growing season (Jaeger et al. 1999). Thus, the strong seasonality in substrate supply and enzyme activities seen in this and other studies (Bonnett et al. 2006; Wallenstein et al. 2009; Bell et al. 2010) seems to be a general characteristic of cold and temperate climates. More studies in Mediterranean, (semi-) arid and tropical environments would help to determine if similar patterns exist in warmer biomes.
While we have focussed on the role of substrate supply, other environmental factors could potentially produce the observed seasonal patterns in enzyme measurements. Firstly, variations in soil moisture have been related to enzyme activity measurements in other systems (Henry 2012). At our site, soil moisture showed only small declines (from 88.0 to 86.9 % by weight) over the entire study period, a fluctuation that is probably not sufficient to explain the patterns we observed. Secondly, soil temperature increased over the course of the growing season (Fig. 1) and could be invoked to explain the seasonal changes in enzyme potential activity. However, we propose that it is more likely that temperature acts on enzyme pools indirectly, i.e. by driving the seasonality of carbon and nutrient availability as discussed above. Experimental incubations of peat soils showed that enzyme pools were much more sensitive to substrate supply than to even a 5 °C increase in incubation temperature (Weedon et al. 2013), and, as previously noted, experimental field studies in a range of systems (including the present study) have failed to find a straightforward relationship between temperature and potential enzyme activity (Bell et al. 2010; Henry 2012).
As well as providing evidence for adaptive control over enzyme production (see above), our observation of contrasting shifts in the temperature sensitivity of Vmax over the season may complicate our understanding of warming effects on soil stoichiometric balance. Previous studies have found a higher temperature sensitivity of glycosidases compared to peptidases, and suggested that this discrepancy could have consequences for the balance between C and N cycles under global warming (Koch et al. 2007; Wallenstein et al. 2009; Wallenstein et al. 2011). Our results suggest that the relative temperature sensitivity of C-/N-related processes may shift across the growing season, and thus extrapolating to global warming effects will require more detailed knowledge about the timing of important “hot moments” in soil C and N cycling.
Implications of contrasting temporal patterns in C and N enzymes
As with all studies based on a single environment, observations in a broader range of ecosystems are needed to test the generality of the patterns we have observed. This may be particularly relevant in this case given the somewhat atypical nature of peatland soils: low pH and nutrient availability, high organic matter content and lack of a mineral matrix (Vitt 2006). However, if the seasonal patterns in enzyme pool sizes and composition we have described are found to be more general, it follows that there are some important implications for the interpretation of soil enzyme measurements, and our understanding of the relationship between those measurements and the stoichiometry of soil nutrient and energy cycles.
Recent models for soil carbon and nitrogen dynamics that include microbial biomass and enzyme production assume that enzyme production is a function of microbial biomass, and usually do not distinguish between enzymes produced to acquire carbon/energy, and those produced to acquire nutrients (e.g. nitrogen and phosphorus) (Schimel and Weintraub 2003; Moorhead and Sinsabaugh 2006; Allison et al. 2010). This assumption is supported by recent global syntheses of enzyme activity measurements in soils and sediments which show a convergent 1:1:1 stoichiometry of allocation to enzymes acquiring C, N and P (Sinsabaugh et al. 2008; Sinsabaugh et al. 2009). However, the extent to which this relationship applies to the small spatial and temporal scales relevant to local nutrient cycling remains an open question. Patterns of enzyme stoichiometry that emerge from such global, intersite comparisons over large gradients may be dominated by top-level (e.g. climate, productivity) constraints on enzyme production, and mask significant divergence at the local scale—i.e. within sites, and across seasons—such as that observed in the present study.
Although parsimonious and tractable model frameworks are important, if the seasonal divergence we have observed is more widespread, strategies for incorporating differential potential activity and temperature sensitivity of enzyme functional groups should be developed and their influence on model behaviour assessed. The recently published EEZY model of Moorhead et al. (2012), and DEMENT model of Allison (2012) are examples of approaches that model separate enzyme pools for C and N acquisition (roughly corresponding to the glycosidases and peptidases measured in the present study). These models are able to successfully re-create observed patterns of enzyme dynamics and decomposition rates. Extending this approach to include empirically calibrated temporal variation in environmental drivers and substrate supply could be a useful means for exploring drivers of the seasonal dynamics of soil processes and, in turn, identify critical questions for further field investigations. Such a process of constant feedback between empirical observation and modelling efforts (as exemplified by this special issue) holds the potential to yield great progress in illuminating the role of soil enzymes in soil functioning, and their response to global change.
Conclusions
We found no evidence for effects of increased soil temperatures on the potential activities or temperature sensitivities of glycosidases and peptidases in a sub-arctic peatland, despite previously observed large effects of higher temperatures on respiration and N cycling. On the other hand, we saw a strong seasonal pattern in both enzymatic parameters with opposite seasonal trends for the two functional groups. Our results suggest that warming effects on soil processes in this globally important carbon sink are more likely to act indirectly via patterns in substrate supply, rather than directly via changes to the production of soil enzymes. Furthermore, the observed divergence of glycosidase and peptidase enzyme activities and temperature sensitivities on a seasonal timescale suggest that modelling approaches that treat these functional groups separately and partly independently should be further explored. Deeper mechanistic understanding of the complex interplay of C and N cycles in soil ecosystems will allow more robust predictions of the response of these systems to climate change.
References
ACIA (2004) Impacts of a Warming Arctic: Arctic Climate Impact Assessment. Cambridge University Press, Cambridge
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R, Cornelissen JHC, van Logtestijn RSP, Callaghan TV (2007) Climate change has only a minor impact on nutrient resorption parameters in a high-latitude peatland. Oecologia 151:132–139
Aerts R, Callaghan TV, Dorrepaal E, van Logtestijn RSP, Cornelissen JHC (2012) Seasonal climate manipulations have only minor effects on litter decomposition rates and N dynamics but strong effects on litter P dynamics of sub-arctic bog species. Oecologia 170:809–819
Allison SD (2012) A trait-based approach for modelling microbial litter decomposition. Ecol Lett 15:1058–1070
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA 105:11512–11519
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Allison SD, Gartner TB, Holland K, Weintraub M, Sinsabaugh RL (2007) Soil enzymes: linking proteomics and ecological process. In: Lipson DA, Mills AL, Stetzenbach LD (eds) Manual of Environmental Microbiology, 3rd edn. ASM Press, Herndon, pp 704–711
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Allison SD, Weintraub M, Gartner TB, Waldrop MP (2011) Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In: Shukla G, Varma A (eds) Soil enzymology. Springer-Verlag, Berlin
Bell TH, Henry HAL (2011) Fine scale variability in soil extracellular enzyme activity is insensitive to rain events and temperature in a mesic system. Pedobiologia 54:141–146
Bell TH, Klironomos JN, Henry HAL (2010) Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Sci Soc Am J 74:820–828
Bonnett SAF, Ostle N, Freeman C (2006) Seasonal variations in decomposition processes in a valley-bottom riparian peatland. Sci Total Environ 370:561–573
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Chapman SJ, Thurlow M (1998) Peat respiration at low temperatures. Soil Biol Biochem 30:1013–1021
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol Biochem 41:1180–1186
Dorrepaal E, Aerts R, Cornelissen JHC, Callaghan TV, van Logtestijn RSP (2004) Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Global Change Biol 10:93–104
Dorrepaal E, Toet S, van Logtestijn RSP, Swart E, van de Weg MJ, Callaghan TV, Aerts R (2009) Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:U616–U679
Falge E, Baldocchi D, Tenhunen J, Aubinet M, Bakwin P, Berbigier P, Bernhofer C, Burba G, Clement R, Davis KJ, Elbers JA, Goldstein AH, Grelle A, Granier A, Guomundsson J, Hollinger D, Kowalski AS, Katul G, Law BE, Malhi Y, Meyers T, Monson RK, Munger JW, Oechel W, Paw KT, Pilegaard K, Rannik U, Rebmann C, Suyker A, Valentini R, Wilson K, Wofsy S (2002) Seasonality of ecosystem respiration and gross primary production as derived from FLUXNET measurements. Agric For Meteorol 113:53–74
Finzi AC, Austin AT, Cleland EE, Frey SD, Houlton BZ, Wallenstein MD (2011) Responses and feedbacks of coupled biogeochemical cycles to climate change: examples from terrestrial ecosystems. Front Ecol Environ 9:61–67
Freeman C, Ostle N, Kang H (2001) An enzymic ‘latch’ on a global carbon store—a shortage of oxygen locks up carbon in peatlands by restraining a single enzyme. Nature 409:149
German DP, Chacon SS, Allison SD (2011) Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 92:1471–1480
Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59
Jaeger CH, Monson RK, Fisk MC, Schmidt SK (1999) Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80:1883–1891
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Keuper F, Dorrepaal E, Van Bodegom P, Aerts R, van Logtestijn RSP, Callaghan TV, Cornelissen JHC (2011) A race for space? How Sphagnum fuscum stabilizes vegetation composition during long-term climate manipulations. Global Change Biol 17:2162–2171
Knicker H (2011) Soil organic N—an under-rated player for C sequestration in soils? Soil Biol Biochem 43:1118–1129
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Global Biogeochem Cycles. doi:10.1029/2007GB002983
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Limpens J, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G (2008) Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5:1475–1491
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol Biochem 53:133–141
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology and applications. Marcel Dekker, New York, pp 1–33
Rosswall T, Granhall U (1980) Nitrogen cycling in a subarctic ombrotrophic mire. Ecol Bulletins 30:209–234
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) Gcte-news, a meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Schmidt SK, Costello EK, Nemergut DR, Cleveland CC, Reed SC, Weintraub MN, Meyer AF, Martin AM (2007) Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88:1379–1385
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DA, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Sinsabaugh RL, Foreman CM (2003) Integrating dissolved organic matter metabolism and microbial diversity: an overview of conceptual models. In: Findlay SEG, Sinsabaugh RL (eds) Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press, New York
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795
Skujinš J (1976) Extracellular enzymes in soil. Crit Rev Microbiol 4:383–421
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Todd-Brown KEO, Hopkins FM, Kivlin SN, Talbot JM, Allison SD (2012) A framework for representing microbial decomposition in coupled climate models. Biogeochemistry 109:19–33
Trasar-Cepeda C, Gil-Sotres F, Leiros MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol Biochem 39:311–319
Vicca S, Luyssaert S, Penuelas J, Campioli M, Chapin FS, Ciais P, Heinemeyer A, Hogberg P, Kutsch WL, Law BE, Malhi Y, Papale D, Piao SL, Reichstein M, Schulze ED, Janssens IA (2012) Fertile forests produce biomass more efficiently. Ecol Lett 15:520–526
Vitt DH (2006) Functional characteristics and indicators of boreal peatlands. In: Wieder RK, Vitt DH (eds) Boreal peatland ecosystems. Ecological studies. Springer-Verlag, Berlin
Wallenstein M, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Global Change Biol 15:1631–1639
Wallenstein M, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh RL (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil Enzymology. Springer-Verlag, Berlin
Weedon JT, Aerts R, Kowalchuk GA, van Bodegom PM (2011) Enzymology under global change: organic nitrogen turnover in alpine and sub-arctic soils. Biochem Soc Trans 39:309–314
Weedon JT, Kowalchuk GA, Aerts R, van Hal JR, van Logtestijn RKSP, Taş N, Röling WF, van Bodegom PM (2012) Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Global Change Biol 18:138–150
Weedon JT, Aerts R, Kowalchuk G, van Logtestijn RkSP, Andringa D, van Bodegom P (2013) Temperature sensitivity of peatland C and N cycling: does substrate supply play a role? Soil Biol Biochem 69:109–120
Weintraub MN, Schimel JP (2005) Seasonal protein dynamics in Alaskan arctic tundra soils. Soil Biol Biochem 37:1469–1475
Acknowledgments
This study was financially supported by the Netherlands Organisation for Scientific Research (NWO) by NWO-ALW grant 816.01.012 to RA and GAK. We gratefully acknowledge the efforts of J.H.C. Cornelissen, R.S.P. van Logtestijn and E. Dorrepaal in establishing and maintaining the OTC experiment, thank R.S.P. van Logtestijn for field assistance, and J. Kamstra and P. Cenijn for access to the fluorometer. We also are grateful to Abisko Naturvetenskapliga Station for hosting us during fieldwork. The County Administrative Board at Luleå gave permission to perform the field experiments in Abisko National Park. Comments from anonymous reviewers allowed us to improve upon an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Colin Bell
Rights and permissions
About this article
Cite this article
Weedon, J.T., Aerts, R., Kowalchuk, G.A. et al. No effects of experimental warming but contrasting seasonal patterns for soil peptidase and glycosidase enzymes in a sub-arctic peat bog. Biogeochemistry 117, 55–66 (2014). https://doi.org/10.1007/s10533-013-9870-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9870-0