Abstract
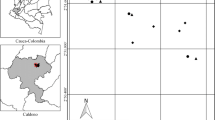
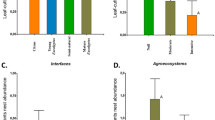
Agriculture of varying management intensity dominates fragmented tropical areas and differentially impacts organisms across and within taxa. We examined impacts of local and landscape characteristics on four groups of ants in an agricultural landscape in Chiapas, Mexico comprised of forest fragments and coffee agroecosystems varying in habitat quality. We sampled ground ants found in leaf litter and rotten logs and arboreal ants found in hollow coffee twigs and on tree trunks. Then using vegetation and agrochemical indices and conditional inference trees, we examined the relative importance of local (e.g. vegetation, elevation, agrochemical) and landscape variables (e.g. distance to and amount of nearby forest and rustic coffee) for predicting richness and abundance of ants. Leaf litter ant abundance increased with vegetation complexity; richness and abundance of ants from rotten logs, twig-nests, and tree trunks were not affected by vegetation complexity. Agrochemical use did not affect species richness or abundance of any ant group. Several local factors (including humus mass, degree of decay of logs, number of hollow twigs, tree circumference, and absence of fertilizers) were significant positive predictors of abundance and richness of some ant groups. Two landscape factors (forest within 200 m, and distance from forest) predicted richness and abundance of twig-nesting and leaf litter ants. Thus, different ant groups were influenced by different characteristics of agricultural landscapes, but all responded primarily to local characteristics. Given that ants provide ecosystem services (e.g. pest control) in coffee farms, understanding ant responses to local and landscape characteristics will likely inform farm management decisions.



Similar content being viewed by others
References
Agosti D, Alonso LE (2000) The ALL Protocol, a standard protocol for the collection of ground-dwelling ants. In: Agosti D, Majer JD, Alonso LE, Shultz TR (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington, DC, pp 204–206
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2:291–298
Andersen AN, Hoffmann BD, Muller WJ, Griffiths AD (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17
Armbrecht I, Perfecto I (2003) Litter-twig dwelling ant species richness and predation potential within a forest fragment and neighboring coffee plantations of contrasting habitat quality in Mexico. Agric Ecosyst Environ 97:107–115
Armbrecht I, Rivera L, Perfecto I (2005) Reduced diversity and complexity in the leaf-litter ant assemblage of Colombian coffee plantations. Conserv Biol 19:897–907
Batáry P, Matthiesen T, Tscharntke T (2010) Landscape-moderated importance of hedges in conserving farmland bird diversity of organic versus conventional croplands and grasslands. Biol Conserv 143:2020–2027
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300
Bestelmeyer BT, Wiens JA (1996) The effects of land use on the structure of ground-foraging ant communities in the Argentine Chaco. Ecol Appl 6:1225–1240
Bestelmeyer BT, Agosti D, Alonso LE, Brandão CRF, Brown WL, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants. In: Agosti D, Majer JD, Alonso LE, Shultz TR (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington, DC, pp 122–144
Bianchi FJ, Booij CJ, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc B 273:1715–1727
Bisseleua DHB, Missoup AD, Vidal S (2009) Biodiversity conservation, ecosystem functioning, and economic incentives under cocoa agroforestry intensification. Conserv Biol 23:1176–1184
Bivand R, Altman M, Anselin L, Assunção R, Berke O, Bernat A, Blanchet G, Blankmeyer E, Carvalho M, Christensen B, Chun Y, Dormann C, Dray S, Halbersma R, Krainski E, Legendre P, Lewin-Koh N, Li H, Ma J, Millo G, Mueller W, Ono H, Peres-Neto P, Piras G, Reder M, Tiefelsdorf M, Yu D (2012) spdep: Spatial dependence: weighting schemes, statistics and models. R package version 0.5-46. http://CRAN.R-project.org/package=spdep. Accessed Dec 2012
Bjornstad O (2009). ncf: spatial nonparametric covariance functions. R package version 1.1-3. http://CRAN.R-project.org/package=ncf. Accessed Dec 2012
Blüthgen N, Stork NE, Fiedler K (2004) Bottom-up control and co-occurrence in complex communities: honeydew and nectar determine a rainforest ant mosaic. Oikos 106:344–358
Bolton B (1994) Identification guide to the ant genera of the world. Harvard University Press, Cambridge
Brown WL Jr (1976) Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section A. Introduction, subtribal characters. Genus Odontomachus Stud Entomol 19:167–171
Byrne MM (1994) Ecology of twig-dwelling ants in wet lowland tropical forest. Biotropica 6:61–72
Carvalho KS, Vasconcelos HL (1999) Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biol Conserv 91:151–157
Castellon T, Sieving K (2006) An experimental test of matrix permeability and corridor use by an endemic understory bird. Conserv Biol 20:135–145
Chong CS, Hoffmann AA, Thomson LT (2007) Commercial agrochemical applications in vineyards do not influence ant communities. Environ Entomol 36:1374–1383
Clergue B, Amiaud B, Pervanchon F, Lasserre-Joulin F, Plantureux S (2005) Biodiversity: function and assessment in agricultural areas A review. Agron Sustain Dev 25:1–15
Concepción EC, Díaz M, Baquero RA (2008) Effects of landscape complexity on the ecological effectiveness of agri-environment schemes. Landsc Ecol 23:135–148
Connell JH (1978) Diversity in tropical rain forest and coral reefs. Science 199:1302–1310
Daily GC, Ehrlich PR, Sánchez-Azofeifa A (2001) Countryside biogeography: use of human-dominated habitats by the avifauna of southern Costa Rica. Ecol Appl 11:1–13
Dauber J, Hirsch M, Simmering D, Waldhardt R, Otte A, Wolters V (2003) Landscape structure as an indicator of biodiversity: matrix effects on species richness. Agric Ecosyst Environ 98:321–329
Dauber J, Purtauf T, Allspach A, Frisch J, Voigtländer K, Wolters V (2005) Local versus landscape controls on diversity: a test using surface-dwelling soil macroinvertebrates of differing mobility. Global Ecol Biogeogr 14:213–221
Davidson DW, Cook SC, Snelling RR, Chua TH (2003) Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300:969–972
De la Mora A, Philpott SM (2010) Wood-nesting ants and their parasites in forests and coffee agroecosystems. Environ Entomol 39:1473–1481
Dormann CF, McPherson JM, Araújo MB, Bivand R, Bolliger J, Carl G, Davies RG, Hirzel A, Jetz W, Kissling WD, Kühn I, Ohlemüller R, Peres-Neto PR, Reineking B, Schröder B, Schurr FM, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628
Duffy JE (2009) Why biodiversity is important to the functioning of real-world ecosystems. Front Ecol Environ 7:437–444
Ellison AM, Record S, Arguello A, Gotelli NJ (2007) Rapid inventory of the ant assemblage in a temperate hardwood forest: species composition and assessment of sampling methods. Environ Entomol 36:766–775
Gabriel D, Sait SM, Hodgson JA, Schmutz U, Kunin WE, Benton TG (2010) Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecol Lett 13:858–869
García Estrada C, Damon A, Sánchez Hernández C, Soto Pinto L, Guillermo Ibarra-Núñez G (2006) Bat diversity in montane rainforest and shaded coffee under different management regimes in southeastern Chiapas, Mexico. Biol Conserv 132:351–361
Gibb H, Hjältén J, Ball JP, Atlegrim O, Pettersson RB, Hilszczanski J, Johansson T, Danell K (2006) Effects of landscape composition and substrate availability on saproxylic beetles in boreal forests: a study using experimental logs for monitoring assemblages. Ecography 29:191–204
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates, Inc., Sunderland
Gotelli NJ, Ellison AM, Dunn RR, Sanders NJ (2011) Counting ants (Hymenoptera: formicidae): biodiversity sampling and statistical analysis for myrmecologists. Myrmecol News 15:13–19
Hahn D, Wheeler DE (2002) Seasonal foraging activity and bait preferences of ants on Barro Colorado Island, Panama. Biotropica 34:348–356
Hoffmann BD (2010) Using ants for rangeland monitoring: global patterns in the responses of ant communities to grazing. Ecol Indic 10:105–111
Hölldobler B, Wilson EO (1990) The Ants. Springer, Berlin
Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 15:651–674
Jha S, Vandermeer JH (2010) Impacts of coffee agroforestry management on tropical bee communities. Biol Conserv 143:1423–1431
Jules E, Shahani P (2003) A broader ecological context to habitat fragmentation: why matrix habitat is more important than we though. J Veg Sci 14:459–464
Kaspari M (1996) Testing resource-based models of patchiness in four neotropical litter ant assemblages. Oikos 76:443–454
Kaspari M, Weiser MD (2000) Ant activity along moisture gradients in a neotropical forest. Biotropica 32:703–711
Kaspari M, Yanoviak SP (2001) Bait use in tropical litter and canopy ants—evidence of differences in nutrient limitation. Biotropica 33:207–211
Klimes P, Idigel C, Rimandai M, Fayle TM, Janda M, Weiblen GD, Novotny V (2012) Why are there more arboreal ant species in primary than in secondary tropical forests? J Anim Ecol 81:1103–1112
Longino JT (2009) Additions to the taxonomy of New World Pheidole (Hymenoptera: formicidae). Zootaxa 2181:1–90
Longino JT (2011) Ants of Costa Rica.http://academic/evergreen.edu/projects/ants/AntsofCostaRica.html. Accessed January, 2011
Matlock RB, de la Cruz R (2003) Ants as indicators of pesticide impacts in banana. Environ Entomol 32:816–829
McGlynn T, Fawcett R, Clark D (2009) Litter biomass and nutrient determinants of ant density, nest size, and growth in a Costa Rican tropical wet forest. Biotropica 2:234–240
Moguel P, Toledo V (1999) Biodiversity conservation in traditional coffee systems of Mexico. Conserv Biol 13:11–21
Moorhead LC, Philpott SM, Bichier P (2010) Epiphyte biodiversity in the coffee agricultural matrix: canopy stratification and distance from forest fragments. Conserv Biol 24:737–746
Økland B, Bakke A, Hagvar S, Kvamme T (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodiv Conserv 5:75–100
Olden JD, Lawler JJ, Poff NL (2008) Machine learning methods without tears: a primer for ecologists. Q Rev Biol 83:171–192
Oliver I, Garden D, Greenslade PJ, Haller B, Rodgers D, Seeman O, Johnston B (2005) Effects of fertiliser and grazing on the arthropod communities of a native grassland in south-eastern Australia. Agric Ecosyst Environ 109:323–334
Peck SL, McQuaid B, Lee C (1998) Using ant species (Hymenoptera: formicidae) as a biological indicator of agroecosystem condition. Environ Entomol 27:1102–1110
Perfecto I, Snelling R (1995) Biodiversity and the transformation of a tropical agroecosystem: ants in coffee plantations. Ecol Appl 5:1084–1097
Perfecto I, Vandermeer J (1996) Microclimatic changes and the indirect loss of ant diversity in a tropical agroecosystem. Oecologia 108:577–582
Perfecto I, Vandermeer J (2002) Quality of agroecological matrix in a tropical montane landscape: ants in coffee plantations in Southern Mexico. Conserv Biol 16:174–182
Perfecto I, Rice R, Greenberg R, Van der Voort M (1996) Shade coffee: a disappearing refuge for biodiversity. Bioscience 46:598–608
Perfecto I, Vandermeer J, Mas A, Soto Pinto L (2005) Biodiversity, yield, and shade coffee certification. Ecol Econ 54:435–446
Petal J (1980) Ant populations, their regulation and effect on soil in meadows. J Pol Sci 28:297–326
Philpott SM (2005) Changes in arboreal ant populations following pruning of coffee shade-trees in Chiapas, Mexico. Agroforest Syst 64:219–224
Philpott SM, Foster PF (2005) Nest-site limitation in coffee agroecosystems: artificial nests maintain diversity of arboreal ants. Ecol Appl 5:1478–1485
Philpott SM, Arendt W, Armbrecht I, Bichier P, Dietsch T, Gordon C, Greenberg R, Perfecto I, Soto-Pinto L, Tejada-Cruz C, Williams G, Valenzuela J (2008a) Biodiversity loss in Latin American coffee landscapes: reviewing evidence on ants, birds, and trees. Conserv Biol 22:1093–1105
Philpott SM, Lin BB, Jha S, Brines SA (2008b) A multi-scale assessment of hurricane impacts based on land-use and topographic features. Agric Ecosyst Environ 128:12–20
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/
Ricketts T, Daily G, Ehrlich P, Fay J (2001) Countryside biogeography of moths in a fragmented landscape: biodiversity in native and agricultural habitats. Conserv Biol 15:378–388
Rivera L, Armbrecht I (2005) Diversidad de tres gremios de hormigas en cafetales de sombra, de sol y bosques de Risaralda. Rev Colomb Entomol 31:89–96
Schlick-Steiner BC, Steiner FM, Moder K, Bruckner A, Fiedler K, Christian E (2006) Assessing ant assemblages: pitfall trapping versus nest counting (Hymenoptera, Formicidae). Insect Soc 53:274–281
Schmidt MH, Thies C, Nentwig W, Tscharntke T (2008) Contrasting responses of arable spiders to the landscape matrix at different spatial scales. J Biogeogr 35:157–166
Schonberg LA, Longino J, Nadkarni NM, Yanoviak SP, Gering J (2004) Arboreal ant species richness in primary forest, secondary forest, and pasture habitats of a tropical montane landscape. Biotropica 36:402–440
Shik JZ, Kaspari M (2010) More food, less habitat: how necromass and leaf litter decomposition combine to regulate a litter ant community. Ecol Entomol 35:158–165
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale dependent effects of landscape context on three pollinator guilds. Ecology 831:421–1432
Steffan-Dewenter I, Kessler M, Barkmann J, Bos MM, Buchori D, Erasmi S, Faust H, Gerhold G, Glenk K, Grandstein SR, Guhardja E, Harteveld M, Hertel D, Höhn P, Kappas M, Köhler S, Leuschner C, Maertens M, Marggraf R, Migge-Kleian S, Mogea J, Pitopang R, Schaefer M, Schwarze S, Sporn SG, Steingrebe A, Tjitrosoedirdjo SS, Tjitrosoemito S, Twele A, Weber R, Woltmann L, Zeller M, Tscharntke T (2007) Tradeoffs between income, biodiversity, and ecosystem functioning during tropical rainforest conversion and agroforestry intensification. Proc Natl Acad Sci 104:4973–4978
Strobl C, Hothorn T, Zeileis A (2009) Party on! The R Journal 2:14–17
Teodoro AV, Sousa-Souto L, Klein AM, Tscharntke T (2010) Seasonal contrasts in the response of coffee ants to agroforestry shade-tree management. Environ Entomol 39:1744–1750
Torres JA (1994) Wood decomposition of Cyrilla racemiflora in a tropical montane forest. Biotropica 26:124–140
Tscharntke T, Klein A, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol Lett 8:857–874
Tscharntke T, Tylianakis JM, Rand TA, Didham RK, Fahrig L, Batáry P, Bengtsson J, Clough Y, Crist TO, Dormann CF, Ewers RM, Fründ J, Holt RD, Holzschuh A, Klein AM, Kleijn D, Kremen C, Landis DA, Laurance W, Lindenmayer D, Scherber C, Sodhi N, Steffan-Dewenter I, Thies C, van der Putten WH, Westphal C (2012) Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biol Rev Camb Philos Soc 87:661–685
Tylianakis JM, Klein AM, Tscharntke T (2005) Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 86:3296–3302
Uno S, Cotton J, Philpott SM (2010) Diversity and species composition of ants in urban green spaces. Urban Ecosyst 13:425–441
Vandermeer J, Carvajal R (2001) Metapopulation dynamics and the quality of the matrix. Am Nat 159:211–220
Vasconcelos HL, Laurance WF (2005) Influence of habitat, litter type, and soil invertebrates on leaf-litter decomposition in a fragmented Amazonian landscape. Oecologia 144:456–462
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th edn. Springer, New York. ISBN 0-387-95457-0
Acknowledgments
A. García Ballinas, J. Santis, G. Dominguez, U. Pérez Vásquez, G. López Bautista, B.E. Chilel, E. Schüller, S. Arming, and E. Sintes assisted with field work. R. Becker, R. John, and J.H. López Urbina assisted with the GIS analysis. B. Nickel provided assistance with spatial analysis. G. Ibarra Núñez, J. Rojas, J. Valle-Mora, and E. Chamé Vásquez of El Colegio de la Frontera Sur (ECOSUR) provided logistical support. C. Hochreiter, D. Gonthier, K. Ennis, G. Ibarra Núñez, J.-P. Lachaud, G. Pérez-Lachaud, L. Soto-Pinto, D. Allen, D. Jackson and J. Remfert provided comments on the manuscript. We thank the owners of Fincas Irlanda, Argovia, Hamburgo, San Francisco, Genova, Rancho Alegre, Chiripa, Maravillas, Santa Anita, San Enrique and the Rogers Family Company for allowing us to conduct research on their farms. Finca Irlanda and Don Walter Peters provided housing. CM was funded by University of Toledo Undergraduate Summer Research and Creative Activity Program, A Study Abroad Travel Grant, and the Explorer’s Club Youth Activity Fund. ADM was funded by scholarship number 168970 granted by the National Council of Science and Technology (CONACYT) in Mexico and a Conservation International Rapid Assessment Program award. Additional funding was provided by NSF DEB-1020096 to SMP.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De la Mora, A., Murnen, C.J. & Philpott, S.M. Local and landscape drivers of biodiversity of four groups of ants in coffee landscapes. Biodivers Conserv 22, 871–888 (2013). https://doi.org/10.1007/s10531-013-0454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-013-0454-z