Abstract
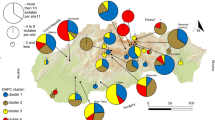
Field surveys in 2006 confirmed that the exotic rust fungus Phragmidium violaceum was widespread on Rubus armeniacus and Rubus laciniatus in the Pacific Northwest of the USA. The origin and dispersal pattern of this obligate biotrophic pathogen in the USA were investigated by comparing the genetic diversity and structure of 27 isolates each from the USA and Europe, and 20 isolates from Australia where an invasion occurred in 1984. Analysis of 11 microsatellite loci revealed 74 unique genotypes, with the European population having a significantly higher level of allelic diversity and number of private alleles compared to populations from the USA and Australia. Principal coordinate analysis (PCA), analysis of molecular variance and pairwise comparisons of Φ confirmed a strong level of differentiation among continental populations, with little divergence between isolates from the USA and Europe, but a high level of differentiation between these isolates and those from Australia. These results were broadly supported by the Bayesian cluster analysis, which indicated that at K = 3 the clustering of the isolates corresponds to their geographic origin. Bayesian clustering, PCA as well as insignificant migration estimates from Europe to the USA suggest that the USA population is not a direct descendant from the European P. violaceum population. There was a weak association between genetic and geographic distance among the USA isolates, suggesting invasion was initially localized prior to dispersal or that the population may have been present for some time prior to first detection in 2005.






Similar content being viewed by others
References
Aime MC, Rossman AY (2007) First report of the rust Phragmidium violaceum on Pennsylvania blackberry in California. Plant Dis 91:1517
Brasier CM, Buck KW (2001) Rapid evolutionary changes in a globally invading fungal pathogen (Dutch Elm Disease). Biol Invasions 3:223–233
Brown JKM, Hovmøller MS (2002) Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297:537–541
Brown AHD, Weir BS (1983) Measuring genetic variability in plant populations. In: Tanksley SD, Orton TJ (eds) Isozymes in plant genetics and breeding, part A. Elsevier Science Publ., Amsterdam, pp 219–239
Bruzzese E, Field RP (1985) Occurrence and spread of Phragmidium violaceum on blackberry (Rubus fruticosus) in Victoria, Australia. In: Delfosse ES (ed) Proceedings of the VI international symposium on biological control of weeds. Agriculture Canada, Ottawa, pp 609–612
Bruzzese E, Hasan S (1986) The collection and selection in Europe of isolates of Phragmidium violaceum (Uredinales) pathogenic to species of European blackberry naturalized in Australia. Ann Appl Biol 108:527–533
Clark LV, Evans KJ, Jasieniuk M (2013) Origins and distribution of invasive Rubus fruticosus L. agg. (Rosaceae) clones in the Western United States. Biol Invasions. doi:10.1007/s10530-012-0369-8
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Cullen JM, Kable PF, Catt M (1973) Epidemic spread of a rust imported for biological control. Nature 244:462–464
Desprez-Loustau M-L, Robin C, Buée M, Courtecuisse R, Garbaye J, Suffert F, Sache I, Rizzo DM (2007) The fungal dimension of biological invasions. Trends Ecol Evol 22:472–480
DiTomaso JM, Healy EA (2007) Weeds of California and Other Western States. University of California Division of Agriculture and Natural Resources, Oakland
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Dutech C, Fabreguettes O, Capdevielle X, Robin C (2010) Multiple introduction of divergent genetic lineages in an invasive fungal pathogen, Cryphonectria parasitica, in France. Heredity 105:220–228
Estoup A, Guillemaud T (2010) Reconstructing routes of invasion using genetic data: why, how and so what? Mol Ecol 19:4113–4130
Estoup A, Jarne P, Cornuet J-M (2002) Homoplasy and mutation model at microsatellite loci and their consequences for population genetics analysis. Mol Ecol 11:1591–1604
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Evans KJ, Weber HE (2003) The most widespread taxon of weedy blackberry in Australia is Rubus anglocandicans (Rosaceae). Aust Syst Bot 16:527–537
Evans KJ, Jones MK, Mahr FA, Roush RT (2000) DNA phenotypes of the blackberry biological control agent, Phragmidium violaceum, in Australia. Australas Plant Pathol 29:249–254
Evans KJ, Symon DE, Whalen MA, Hosking JR, Barker RM, Oliver JA (2007) Systematics of the Rubus fruticosus aggregate (Rosaceae) and other exotic Rubus taxa in Australia. Aust Syst Bot 20:187–251
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Farr DF, Rossman AY (2012) Fungal databases, systematic mycology and microbiology laboratory. ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/. Accessed 30 Mar 2012.
Fitzpatrick BM, Fordyce JA, Miller ML, Reynolds RG (2012) What can DNA tell us about biological invasions? Biol Invasions 14:245–253
François O, Durand E (2010) Spatially explicit Bayesian clustering models in population genetics. Mol Ecol Res 10:773–784
Gladieux P, Zhang X-G, Róldan-Ruiz I, Caffier V, Leroy T, Devaux M, Van Glabeke S, Coart E, Le Cam B (2010) Evolution of the population structure of Venturia inaequalis, the apple scab fungus, associated with the domestication of its host. Mol Ecol 19:658–674
Gomez DR, Evans KJ, Harvey PR, Baker J, Barton J, Jourdan M, Morin L, Pennycook SR, Scott ES (2006) Genetic diversity in the blackberry rust pathogen, Phragmidium violaceum, in Europe and Australasia as revealed by analysis of SAMPL. Mycol Res 110:423–430
Gomez DR, Evans KJ, Baker J, Harvey PR, Scott ES (2008) Dynamics of introduced populations of Phragmidium violaceum and implications for biological control of European blackberry in Australia. Appl Environ Microbiol 74:5504–5510
Hey J, Nielsen R (2004) Multilocus methods for estimating population sizes, migration rates and divergence time, with applications to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics 167:747–760
Hey J, Nielsen R (2007) Integration within the Felsenstein equation for improved Markov chain Monte Carlo methods in population genetics. Proc Natl Acad Sci USA 20:2785–2790
Isard SA, Gage SH, Comtois P, Russo JM (2005) Principles of the atmospheric pathway for invasive species applied to soybean rust. Bioscience 55:851–861
Johnson KB, Mahaffee WF (2010) Factors influencing epidemiology and management of blackberry rust in cultivated Rubus laciniatus. Plant Dis 94:581–588
Kimura M, Crow J (1964) The number of alleles that can be maintained in a finite population. Genetics 49:725–738
Laundon GF, Rainbow AF (1969) Phragmidium violaceum (Schultz) Wint. In: Descriptions of pathogenic fungi and bacteria. Commonwealth Mycological Institute, Kew, No. 209
Lessa EP (1990) Multidimensional analysis of geographic genetic structure. Syst Zool 39:242–252
Marks GC, Pascoe IG, Bruzzese E (1984) First record of Phragmidium violaceum on blackberry in Victoria. Australas Plant Pathol 13:12–13
Molecular Ecology Resources Primer Development Consortium, Anderson CM, Aparicio GJ et al (2010) Permanent genetic resources added to molecular ecology resources database 1(December), pp. 2009–31, January 2010. Mol Ecol Res 10:576–579
Morin L, Evans KJ, Jourdan M, Gomez DR, Scott JK (2011) Use of a trap garden to find additional genetically distinct isolates of the rust fungus Phragmidium violaceum to enhance biological control of European blackberry in Australia. Eur J Plant Pathol 131:289–303
Munkacsi A, Stoxen S, May G (2008) Ustilago maydis populations tracked maize through domestication and cultivation in the Americas. Proc R Soc B 275:1037–1046
Nagarajan S, Singh DV (1990) Long-distance dispersion of rust pathogens. Annu Rev Phytopathol 28:139–153
Oehrens EB (1977) Biological control of the blackberry through the introduction of rust, Phragmidium violaceum, in Chile. FAO Plant Protect B 25:26–28
Oehrens E, González S (1974) Introduccion de Phragmidium violaceum (Schulz) Winter como factor de control biologico de zarzamora (Rubus constrictus Lef. et M. y Rubus ulmifolius Schott). Agro Sur 2:30–33
Oehrens E, González S (1977) Dispersion, ciclo biologico y daños causados por Phragmidium violaceum (Schulz) winter en zarzamora (Rubus constrictus Lef. et M. and Rubus ulmifolius Schott.) en las zonas centro-sur y sur de Chile. Agro Sur 5:73–85
Osterbauer N, Trippe A, French K, Butler T, Aime MC, McKemy J, Bruckart WL, Peerbolt T, Kaufman D (2005) First report of Phragmidium violaceum infecting Himalaya and evergreen blackberries in North America. Plant Health Progr. doi:10.1094/PHP-2005-0923-01-BR
Peakall R, Smouse P (2006) Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Piry S, Luikart G, Cornuet JM (1999) Bottleneck: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:503
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Purdy LH, Krupa SV, Dean JL (1985) Introduction of sugarcane rust into the Americas and its spread to Florida. Plant Dis 69:689–693
Robert S, Ravigne V, Zapater M-F, Abadie C, Carlier J (2012) Contrasting introduction scenarios among continents in the worldwide invasion of the banana fungal pathogen Mycosphaerella fijiensis. Mol Ecol 21:1098–1114
Rossman AY (2009) The impact of invasive fungi on agricultural ecosystems in the United States. Biol Invasions 11:97–107
Rousset F (2008) Genepop′007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Notes 8:103–106
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Scott JK, Jourdan M, Evans KJ (2002) Biological control of blackberry: progress towards finding additional strains of the rust fungus, Phragmidium violaceum. In: Spafford Jacob H, Dodd J, Moore JH (eds) Proceedings of the 13th Australian weeds conference. Plant Protection Society of WA Inc., Perth, pp 418–421
Sheridan JE (1989) Quarantine risks imposed by overseas passengers. N Z J For Sci 19:338–346
Smart CD, Fry WE (2001) Invasions by the late blight pathogen: renewed sex and enhanced fitness. Biol Invasions 3:235–243
Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–573
Tykhonenko YY (2007) Geographical distribution of the genus Phragmidium link. Ukr Botan Journ 64:35–41
Walker J, Hartigan D, Bertus AL (1974) Poplar rusts in Australia with comments on potential conifer rusts. Eur J For Pathol 4:100–118
Waples RS, Gaggiotti O (2006) What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol Ecol 15:1419–1439
Washington WS (1991) Blackberry rust. In: Ellis MA, Converse RH, Williams RN, Williamson B (eds) Compendium of raspberry and blackberry diseases and insects. APS Press, St. Paul, pp 32–33
Wright S (1969) Evolution and the genetics of populations. University of Chicago Press, Chicago
Acknowledgments
We thank Mireille Jourdan (CSIRO Ecosystem Sciences, Montpellier) for providing the European isolates of P. violaceum used in the study, Patricia Wallace and Andrew Albrecht (USDA-ARS, Corvallis, Oregon) for assistance in collecting the USA isolates, and Diana Hartley (CSIRO Ecosystem Sciences, Canberra) for helpful guidance and discussion. We also thank Dr Wee Tek Tay (CSIRO Ecosystem Sciences, Canberra) and Dr Niklaus Grünwald (USDA-ARS Horticulture Crops Research Unit Corvallis, OR), for comments on an earlier draft of the paper. Financial support from the Australian Government, Department of Agriculture, Fisheries and Forestry and USDA ARS Specific Cooperative Agreement 58-5358-5-793, which was awarded through the Northwest Center for Small Fruits Research, and USDA-ARS CRIS 5358-22000-034-00 is gratefully acknowledged. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morin, L., Gomez, D.R., Evans, K.J. et al. Invaded range of the blackberry pathogen Phragmidium violaceum in the Pacific Northwest of the USA and the search for its provenance. Biol Invasions 15, 1847–1861 (2013). https://doi.org/10.1007/s10530-013-0413-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0413-3