Abstract
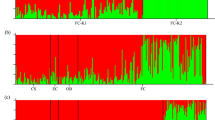
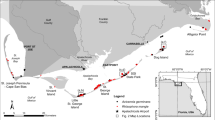
Two species of mangrove trees of Indo-Pacific origin have naturalized in tropical Atlantic mangrove forests in South Florida after they were planted and nurtured in botanic gardens. Two Bruguiera gymnorrhiza trees that were planted in the intertidal zone in 1940 have given rise to a population of at least 86 trees growing interspersed with native mangrove species Rhizophora mangle, Avicennia germinans and Laguncularia racemosa along 100 m of shoreline; the population is expanding at a rate of 5.6% year−1. Molecular genetic analyses confirm very low genetic diversity, as expected from a population founded by two individuals. The maximum number of alleles at any locus was three, and we measured reduced heterozygosity compared to native-range populations. Lumnitzera racemosa was introduced multiple times during the 1960s and 1970s, it has spread rapidly into a forest composed of native R. mangle, A. germinans, Laguncularia racemosa and Conocarpus erectus and now occupies 60,500 m2 of mangrove forest with stem densities of 24,735 ha−1. We estimate the population growth rate of Lumnitzera racemosa to be between 17 and 23% year−1. Populations of both species of naturalized mangroves are dominated by young individuals. Given the long life and water-dispersed nature of propagules of the two exotic species, it is likely that they have spread beyond our survey area. We argue that the species-depauperate nature of tropical Atlantic mangrove forests and close taxonomic relatives in the more species-rich Indo-Pacific region result in the susceptibility of tropical Atlantic mangrove forests to invasion by Indo-Pacific mangrove species.





Similar content being viewed by others
References
Abrantes K, Sheaves M (2009) Food web structure in a near-pristine mangrove areas of the Australian wet tropics. Estuar Coast Shelf Sci 82:597–607
Allen JA (1998) Mangroves as alien species: the case of Hawaii. Glob Ecol Biogeogr Lett 7:61–71
Allen JA, Duke NC (2006) Species profiles for Pacific Island Agroforestry. http://www.traditionaltree.org. Accessed 3 August 2009
Allen JA, Krauss KW (2006) Influence of propagule flotation longevity and light availability on establishment of introduced mangrove species in Hawai’i. Pac Sci 60:367–376
Biswas SR, Choudhury JK, Nishat A, Rahman MM (2007) Do invasive plants threaten the Sundarbans mangrove forest of Bangladesh? For Ecol Manag 245:1–9
Bosire JO, Kairo JG, Kazungu J, Koedam N, Dahdouh-Guebas F (2005) Predation on propagules regulates regeneration in a high-density reforested mangrove plantation. Mar Ecol Prog Ser 299:149–155
Chen X, Lin P, Lin Y (1996) Mating system and spontaneous mutation rates for chlorophyll deficiency in populations of the mangrove Kandelia candel. Hereditas 125:47–52
Chimner RA, Fry B, Kaneshiro MY, Cormier N (2006) Current extent and historical expansion of introduced mangroves on O’ahu, Hawai’i. Pac Sci 60:377–383
Clarke WC, Thaman RR eds (1993) Agroforestry in the Pacific Islands: systems for sustainability. United Nations University Press, Tokyo, 297 pp
Clarke PJ, Kerrigan RA (2002) The effects of seed predators on the recruitment of mangroves. J Ecol 90:728–736
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Cox EF, Allen JA (1999) Stand structure and productivity of the introduced Rhizophora mangle in Hawaii. Estuaries 22:276–284
Demopoulos AWJ, Fry B, Smith CR (2007) Food web structure in exotic and native mangroves: a Hawaii—Puerto Rico comparison. Oecologia 153:675–686
Donnelly MJ, Green DM, Walters LJ (2008) Allelopathic effects of the fruits of the Brazillian perpper Schinus terebinthifolius on growth, leaf production and biomass of seedlings of the red mangrove Rhizophora mangle and the black mangrove Avicennia germinans. J Exp Mar Biol Ecol 357:149–156
Doren RF, Jones DT (1997) Management in Everglades National Park. In: Simberloff D, Schmitz DC, Brown TC (eds) Strangers in Paradise: impact and management of nonindigenous species in Florida. Island Press, Washington, DC, 467 pp
Duke NC (1992) Mangrove floristics and biogeography. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, DC, pp 63–100
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Fairchild D (1898) Systematic plant introduction: its purposes and methods. Bulletin #21, Division of Forestry, US Department of Agriculture, Government Printing Office, Washington, 21 pp
Fairchild D (1945) Garden Islands of the Great East. Charles Scribners, New York, 239 pp
Gillis WT (1971) A Bruguiera Party. Kampong Notes 6(3), 30 September 1971
Gordon DR (1998) Effects of invasive, non-indigenous plant species on ecosystem processes: lessons from Florida. Ecol Appl 8:975–989
Graham A (2006) Paleobotanical evidence and molecular data in reconstructing the historical phytogeography of the Rhizophoraceae. Ann Mo Bot Gard 93:325–334
Harper JL (1965) Establishment, aggression and cohabitation. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic Press, New York, pp 243–265
He BY, Lai TH, Fan HQ, Wang WQ, Zheng HL (2007) Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the beibu Gulf. Estuar Coast Shelf Sci 74:254–262
Imai N, Takyu M, Nakamura Y, Nakamura T (2006) Gap formation and regeneration of tropical mangrove forests in Ranong, Thailand. Plant Ecol 186:37–46
Islam MS, Lian CL, Kameyama N, Wu B, Hogetsu T (2006) Development and characterization of ten new microsatellite markers in a mangrove tree species Bruguiera gymnorrhiza (L.). Mol Ecol Notes 6:30–32
Jones DT, Doren RF (1997) The distribution, biology and control of Schinus terebinthifolius in Southern Florida, with special reference to Everglades National Park. In: Brock JH, Wade M, Pysek P, Green D (eds) Plant invasions: studies from North America and Europe. Backhuys, Leiden, pp 81–93
Krauss KW, Allen JA (2003a) Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquat Bot 77:311–324
Krauss KW, Allen JA (2003b) Factors influencing the regeneration of the mangrove Bruguiera gymnorrhiza (L.) Lamk. on a tropical Pacific Island. For Ecol Manag 176:49–60
Langer MR, Lipps JH (2006) Assembly and persistence of foraminifera in introduced mangroves on Moorea, French Polynesia. Micropaleontology 52:343–355
Lugo AE (1998) Mangrove forests: a tough system to invade but an easy one to rehabilitate. Mar Pollut Bull 37:427–430
Lugo AE, Zucca CP (1977) The impact of low temperature stress on mangrove structure and growth. Trop Ecol 18:149–161
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Maguire TL, Saenger P, Baverstock P, Henry R (2000) Microsatellite analysis of genetic structure in the mangrove species Avicennia marina (Forsk.) Viehr. (Avicenniaceae). Mol Ecol 9:1853–1862
McCoy ED, Heck KL Jr (1976) Biogeography of corals, seagrasses, and mangroves: an alternative to the center of origin concept. Syst Zool 25:201–210
Meziane T, d’Agata F, Lee SY (2006) Fate of mangrove organic matter along a subtropical estuary: small-scale exportation and contribution to the food of mangrove crab communities. Mar Ecol Prog Ser 312:15–27
Moseman SM, Zhang R, Qian PY, Levin LA (2009) Diversity and functional responses of nitrogen-fixing microbes to three wetland invasions. Biol Invasions 11:225–239
Nunez-Farfan J, Dominguez CA, Eguiarte LE, Cornejo A, Quijano M, Vargas J, Dirzo R (2002) Genetic divergence among Mexican populations of red mangrove (Rhizophora mangle): geographic and historic effects. Evol Ecol Res 4:1049–1064
Odum WE, Heald EJ (1975) The detritus-based food web of an estuarine mangrove community. In: Cronin LE (ed) Estuarine research. Academic Press, New York, pp 265–286
Odum WE, McIvor CC, Smith TJ III (1982) The ecology of the mangroves of South Florida: a community profile. FWS/OBS-81/24. US Fish and Wildlife Service, Office of Biological Services, Washington, DC
Peakall R, Smouse P (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pemberton RE, Liu H (2009) Marketing time predicts naturalization of horticultural plants. Ecology 90(1):69–80
Pinzon ZS, Ewel KC, Putz FE (2003) Gap formation and forest regeneration in a Micronesian mangrove forest. J Trop Ecol 19:143–153
Raymond M, Rousset F (1995) GENEPOP (version 3.4): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Smith TJ III (1987) Seed predation in relation to tree dominance and distribution in mangrove forests. Ecology 68:266–273
Smith TJ III (2004) Development of a long-term sampling network to monitor restoration success in the southwest coast everglades: vegetation, hydrology, and sediments. USGS Fact Sheet FS-2004-3015. http://sofia.usgs.gov/publications/fs/2004-3015/
Smith TJ III, Robblee MB, Wanless HR, Doyle TW (1994) Mangroves, hurricanes and lightning strikes. Bioscience 44:256–262
Smith TJ III, Anderson GH, Balentine K, Tiling G, Ward GA, Whelan KRT (2009) Cumulative impacts of hurricanes on Florida mangrove ecosystems: sediment deposition, storm surges and vegetation. Wetlands 29:24–34
Steele OC, Ewel KC, Goldstein G (1999) The importance of propagule predation in a forest of non-indigenous mangrove trees. Wetlands 19:705–708
Stevens PW, Fox SL, Montague CL (2006) The interplay between mangroves and saltmarhes at the transition between temperate and subtropical climate in Florida. Wetlands Ecol Manage 14:435–444
Sweeney K (1967) Kampong Notes 2(6), March 1967
Tomlinson B (1986) The botany of mangroves. Cambridge University Press, Cambridge 419 pp
Walker TD, Valentine JW (1984) Equilibrium models of evolutionary species diversity and the number of empty niches. Am Nat 124:887–889
Ward GA, Smith TJ III, Whelan KRT, Doyle TW (2006) Regional processes in mangrove ecosystems: spatial scaling relationships, biomass, and turnover rates following catastrophic disturbance. Hydrobiologia 569:517–527
Ye Y, Tam NFY, Wong YS, Lu CY (2004) Does sea level rise influence propagule establishment, early growth and physiology of Kandelia candel and Bruguiera gymnorrhiza? J Exp Mar Biol Ecol 306:197–215
Zan QJ, Wang BS, Wang YJ, Li MG (2003) Ecological assessment on the introduced Sonneratia caseolaris and S-apetala at the mangrove forest of Shenzen Bay, China. Acta Bot Sin 45:544–551
Acknowledgments
David T. Jones and the staff at the Kampong of the National Tropical Botanic Garden provided unfettered access to their grounds and helped to document the history of the introduction of B. gymnorrhiza there. Our work would not have been possible without their full cooperation and assistance. We thank Mary Collins for help in obtaining information on non-native mangrove species at FTBG, and a cooperative agreement between FTBG and Miami-Dade County Department of Environmental Resources Management, Environmentally Endangered Lands Program. This study was partially supported by research funds of FTBG allocated to the Plant Molecular Systematics and Conservation Genetics lab jointly operated by FTBG and FIU. The help of K. Balentine, D. Broeksteeg, J. Eells, M. Eygenraam, H. de Groot, F. Scheibler and G. Tiling during field sampling is gratefully acknowledged. This manuscript was improved by the input of four anonymous reviewers. T. J. Smith received financial support from the Terrestrial, Freshwater and Marine Ecosystem Program of the US Geological Survey. This is contribution # 461 of the Southeast Environmental Research Center and contribution # 176 of the Tropical Biology Program at Florida International University. Mention of product and/or trade names does not imply endorsement on the part of the US Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fourqurean, J.W., Smith, T.J., Possley, J. et al. Are mangroves in the tropical Atlantic ripe for invasion? Exotic mangrove trees in the forests of South Florida. Biol Invasions 12, 2509–2522 (2010). https://doi.org/10.1007/s10530-009-9660-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9660-8