Abstract
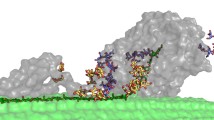
To illustrate the effect of a cellulose-binding domain (CBD) on the enzymatic characteristics of non-cellulolytic exoglucanases, 10 different recombinant enzymes were constructed combining the Saccharomyces cerevisiae exoglucanases, EXG1 and SSG1, with the CBD2 from the Trichoderma reesei cellobiohydrolase, CBH2, and a linker peptide. The enzymatic activity of the recombinant enzymes increased with the CBD copy number. The recombinant enzymes, CBD2-CBD2-L-EXG1 and CBD2-CBD2-SSG1, exhibited the highest cellobiohydrolase activity (17.5 and 16.3 U mg −1 respectively) on Avicel cellulose, which is approximately 1.5- to 2-fold higher than the native enzymes. The molecular organisation of CBD in these recombinant enzymes enhanced substrate affinity, molecular flexibility and synergistic activity, contributing to their elevated action on the recalcitrant substrates as characterised by adsorption, kinetics, thermostability and scanning electron microscopic analysis.
Similar content being viewed by others
References
GW Black JE Rixon JH Clarke GP Hazlewood LM Ferreira DN Bolam HJ Gilbert (1997) ArticleTitleCellulose binding domains and linker sequences potentiate the activity of hemicellulases against complex substrates J. Biotechnol. 57 59–69
C Boisset C Fraschini M Schulein B Henrissat H Chanzy (2000) ArticleTitleImaging the enzymatic digestion of bacterial cellulose ribbons reveals the endo character of the cellobiohydrolase Cel6A from Humicola insolens and its mode of synergy with cellobiohydrolase Cel7A Appl. Environ. Microbiol 66 1444–1452
C Boisset C Pétrequin H Chanzy B Henrissat M Schülein (2001) ArticleTitleOptimized mixtures of recombinant Humicola insolenscellulases for the biodegradation of crystalline cellulose Biotechnol. Bioeng 72 339–345
L Gal S Pages C Gaudin A Belaich C Reverbel-Leroy C Tardif JP Belaich (1997) ArticleTitleCharacterization of the cellulolytic complex (cellulosome) produced by Clostridium cellulolyticum.Appl Environ. Microbiol. 63 903–909
IA Kataeva DL Blum XL Li LG Ljungdahl (2001) ArticleTitleDo domain interactions of glycosyl hydrolases from Clostridium thermocellum contribute to protein thermostability? Protein. Eng. 14 167–172
G Larriba E Andaluz R Cueva RD Basco (1995) ArticleTitleMolecular biology of yeast exoglucanases FEMS Microbiol. Lett. 125 121–126
J Lehtio J Sugiyama M Gustavsson L Fransson M Linder TT Teeri (2003) ArticleTitleThe binding specificity and affinity determinants of family 1 and family 3 cellulose binding modules Proc. Natl. Acad. Sci. USA 100 484–489
M Linder TT Teeri (1997) ArticleTitleThe roles and function of cellulose-binding domains J. Bacteriol 57 15–28
M Linder I Salovuori L Ruohonen TT Teeri (1996) ArticleTitleCharacterization of a double cellulose-binding domain Synergistic high affinity binding to crystalline cellulose. J. Biol. Chem. 271 21268–21272
A Lönn M Gardonyi ZW van HB Hahn R Cordero Otero (2002) ArticleTitleCold adaptation of xylose isomerase from Thermus thermophilus through random PCR mutagenesis Gene cloning and protein characterization. Eur. J. Biochem. 269 IssueID(1 157–163
LR Lynd PJ Weimer WH Van Zyl IS Pretorius (2002) ArticleTitleMicrobial cellulose utilization: fundamentals and biotechnology Microbiol. Mol. Biol. Rev. 66 506–577
DF Malherbe M Du Toit RR Cordero Otero P Van Rensburg IS Pretorius (2003) ArticleTitleExpression of the Aspergillus niger glucose oxidase gene (GOX1) in Saccharomyces cerevisiae and its potential applications in wine production Appl. Microbiol. Biotechnol. 61 502–511
M Srisodsuk T Reinikainen M Penttilä TT Teeri (1993) ArticleTitleRole of the interdomain linker peptide of Trichoderma reesei cellobiohydrolase I in its interaction with crystalline cellulose J. Biol. Chem. 268 20756–20761
K Suzuki T Yabe Y Maruyama K Abe T Nakajima (2001) ArticleTitleCharacterization of recombinant yeast exo-β-1,3-glucanase (Exg1p) expressed in Escherichia coli cells Biosci. Biotechnol. Biochem. 65 1310–1314
P Tomme DP Driver EA Amandoron RC Miller SuffixJr. R Antony J Warren DG Kilburn (1995) ArticleTitleComparison of a fungal (family I) and bacterial (family II) cellulose-binding domain. J. Bacteriol 177 4356–4364
P Van Rensburg WH Zyl Particlevan IS Pretorius (1997) ArticleTitleOver-expression of the Saccharomyces cerevisiae exo-β-1,3-glucanase gene together with the Bacillus subtilis endo-β-1,3 –1,4-glucanase gene and the Butyrivibrio fibrisolvensendo-β-1,4-glucanase gene in yeast J. Biotechnol 55 43–53
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moses, S.B.G., Otero, R.R.C. & Pretorius, I.S. Domain engineering of Saccharomyces cerevisiae exoglucanases. Biotechnol Lett 27, 355–362 (2005). https://doi.org/10.1007/s10529-005-1014-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10529-005-1014-8