Abstract
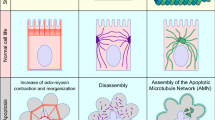
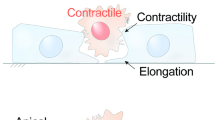
Cellular extrusion is a mechanism that removes dying cells from epithelial tissues to prevent compromising their barrier function. Extrusion occurs in all observed epithelia in vivo and can be modeled in vitro by inducing apoptosis in cultured epithelial monolayers. We established that actin and myosin form a ring that contracts in the surrounding cells that drives cellular extrusion. It is not clear, however, if all apoptotic pathways lead to extrusion and how apoptosis and extrusion are molecularly linked. Here, we find that both intrinsic and extrinsic apoptotic pathways activate cellular extrusion. The contraction force that drives cellular extrusion requires caspase activity. Further, necrosis does not trigger the cellular extrusion response, but instead necrotic cells are removed from epithelia by a passive, stochastic movement of epithelial cells.





Similar content being viewed by others
References
Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA (1994) The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 54:614–617
Gobe GC, Buttyan R, Wyburn KRL, Etheridge MR, Smith PJ (1995) Clusterin expression and apoptosis in tissue remodeling associated with renal regeneration. Kidney Int 47:411–420
Villar CC, Zhao XR (2010) Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol Oral Microbiol 25(3):215–225
Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 11(23):1847–1857
Nagai H, Kalnins VI (1996) Normally occurring loss of single cells and repair of resulting defects in retinal pigment epithelium in situ. Exp Eye Res 62(1):55–61
Peralta Soler A, Mullin JM, Knudsen KA, Marano CW (1996) Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am J Physiol 270(5 Pt 2):F869–F879
Beeman NE, Baumgartner HK, Webb PG, Schaack JB, Neville MC (2009) Disruption of occludin function in polarized epithelial cells activates the extrinsic pathway of apoptosis leading to cell extrusion without loss of transepithelial resistance. BMC Cell Biol 10:85
Pentecost M, Otto G, Theriot JA, Amieva MR (2007) Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoSPathog 2(1):e3
Ninov N, Chiarelli DA, Martín-Blanco E (2007) Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development 134:367–379
Mills JC, Stone NL, Pittman RN (1999) Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol 146(4):703–708
Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D (1999) Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11(3):281–288
Welm AL, Sneddon JB, Taylor C, Nuyten DS, van de Vijver MJ, Hasegawa BH, Bishop JM (2007) The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci USA 104(18):7570–7575
Kepp O, Rajalingam K, Kimmig S, Rudel T (2007) Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J 26(3):825–834
Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE (2007) Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell 128(5):915–929
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17(6):1675–1687
Walczak H, Krammer PH (2000) The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res 256(1):58–66 Review
Debatin KM, Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23:2950–2966
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94(4):481–490
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399(6735):483–487 Erratum in: Nature 407(6805):767
Tsujimoto Y, Shimizu S (2000) VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ 7:1174–1181
Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39(11):615–647
Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A (2007) BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12(2):171–185
Chonghaile TN, Letai A (2008) Mimicking the BH3 domain to kill cancer cells. Oncogene 27(Suppl 1):S149–S157 Review
Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P (1998) Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med 188(5):919–930
Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S (1998) Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol 143(5):1353–1360
Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P (1999) Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol 9(17):967–970
Matsumura H, Shimizu Y, Ohsawa Y, Kawahara A, Uchiyama Y, Nagata S (2000) Necrotic death pathway in Fas receptor signaling. J Cell Biol 151(6):1247–1256
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16(6):663–669 Review
Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, Kroemer G (2000) Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J 14:729–739
Susin SA, Daugas E, Ravagnan L et al (2000) Two distinct pathways leading to nuclear apoptosis. J Exp Med 192:571–580
Loeffler M, Daugas E, Susin SA et al (2001) Dominant cell death induction by extramitochondrially targeted apoptosis-inducing factor. FASEB J 15:758–767
Bröker LE, Kruyt FA, Giaccone G (2005) Cell death independent of caspases: a review. Clin Cancer Res 11(9):3155–3162 Review
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalnotti F, Giazzon M, Dumitiriu IE, Muller S, Iannacone M, Traversari C, Bianchi M, Manfredi AA (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5:825–830
Thorburn J, Frankel AE, Thorburn A (2009) Regulation of HMGB1 release by autophagy. Autophagy 5(2):247–249
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446
Candé C, Cecconi F, Dessen P, Kroemer G (2002) Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115(Pt 24):4727–4734 Review
Tamada M, Perez TD, Nelson WJ, Sheetz MP (2007) Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J Cell Biol 176(1):27–33 Erratum in: J Cell Biol 2007 Feb 12; 176(4):545
Kennedy NJ, Kataoka T, Tschopp J, Budd RC (1999) Caspase activation is required for T cell proliferation. J Exp Med 190:1891
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ (2002) Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419(6905):395–399
Kuranaga E, Miura M (2007) Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol 17(3):135–144 Review
Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, Wallach D (2004) Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol 173(5):2976–2984
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ (2004) Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304(5676):1500–1502
Wang F, Wang F, Zou Z, Liu D, Wang J, Su Y (2010) Active deformation of apoptotic intestinal epithelial cells with adhesion-restricted polarity contributes to apoptotic clearance. Lab Invest 91:462–471
Brancolini C, Benedetti M, Schneider C (1995) Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J 14(21):5179–5190
Sgorbissa A, Benetti R, Marzinotto S, Schneider C, Brancolini C (1999) Caspase-3 and caspase-7 but not caspase-6 cleave Gas2 in vitro: implications for microfilament reorganization during apoptosis. J Cell Sci 112(Pt 23):4475–4482
Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3:346–352
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345
Tomiyoshi G, Horita Y, Nishita M, Ohashi K, Mizuno K (2004) Caspase-mediated cleavage and activation of LIM-kinase 1 and its role in apoptotic membrane blebbing. Genes Cells 9(6):591–600
Kessler T, Müller HA (2009) Cleavage of Armadillo/beta-catenin by the caspase DrICE in Drosophila apoptotic epithelial cells. BMC Dev Biol 9:15
Suzanne M, Steller H (2009) Letting go: modification of cell adhesion during apoptosis. J Biol 8(5):49. Epub 2009 May 28. Review
Bement WM, Forscher P, Mooseker MS (1993) A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol 121(3):565–578
Acknowledgments
We thank Dr. Bryan Welm for use of his microscope, and Drs. Alana Welm, Marco Bianchi, and James Bear for providing the lentiviral and other constructs, and Drs. Karl Matlin and Gruenert for cell lines. We would also like to thank Anna Roth, James Laird and Tony Trinh for their participation and support in this work. This work was supported by a National Institute of Health Innovator Award No. DP2 OD002056-01 to J. Rosenblatt and P30 CA042014 awarded to The Huntsman Cancer Institute for core facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2011_587_MOESM1_ESM.tiff
Figure S1. Etoposide-induced apoptotic dose response curve. MDCK monolayers were treated with increasing concentrations of etoposide (a and b) to determine the concentration at which the percentage of extrusion is maximized without compromising the monolayer integrity. Circles show holes in the monolayers (a) due to the excessive loss via apoptosis, thereby preventing further extrusion. Although, maximal extrusion occurs at 1000 μM etoposide (b), we chose 500 μM etoposide, which was less stressful for the monolayer (a). Scale bars, 10 μm. (TIFF 3363 kb)
10495_2011_587_MOESM2_ESM.tiff
Figure S2. TRAIL-induced apoptotic dose response curve. MDCK monolayers were treated with 100 ng/ml CHX and increasing concentrations of TRAIL (a and b) to determine the concentration at which the percentage of extrusion is maximized without compromising the monolayer integrity. For these analyses, cells were stained for DNA, actin and active caspase-3. The white circle shows a hole in the monolayers (a) due to the excessive loss via apoptosis, preventing extrusion. Although, maximal extrusion occurs at 500 ng/ml TRAIL (b), we chose 100 ng/ml TRAIL, which was less stressful for the monolayer (a). Scale bars, 10 μm. (TIFF 3259 kb)
10495_2011_587_MOESM3_ESM.png
Figure S3. Etoposide-induced apoptotic cell extrusion targets the mitochondrial pathway of apoptosis. MDCK monolayers treated with etoposide were stained for DNA, actin, active caspase-3, and active Bax (a) or cytochrome c (b). The bottom views focus on the actin contractile ring and the top views focus on the cell extruded out of the layer. Arrows point to the apoptotic extruding cells. Scale bars, 10 μm. (PNG 367 kb)
10495_2011_587_MOESM4_ESM.tiff
Figure S4. Downregulation of Bax and Bak blocks cell extrusion induced by an intrinsic apoptosis stimulus. HBE monolayers transduced with either Bax and Bak or non-specific (N.S.) shRNA constructs were treated with UV-C irradiation, and immunostained for DNA, actin, and active caspase-3. Arrows in the top panels show apoptotic extruding cells while arrows in lower panel show the actin contractile rings. Scale bars, 10 μm. (TIFF 2763 kb)
10495_2011_587_MOESM5_ESM.pdf
Figure S5. Time of cell removal. MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with either DMSO or the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (PI) was added to monitor permeability of the dying cells. The graph shows time ranges required for removal of apoptotic and necrotic cell. Error bars represent standard deviation. (PDF 276 kb)
10495_2011_587_MOESM6_ESM.mov
Supplementary Movie 1. Extrusion of an apoptotic cell. MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with DMSO and exposed to UV-C irradiation were timelapsed every 10 min for 12 h. Propidium iodide (red) labels permeable dying cells. (MOV 398 kb)
10495_2011_587_MOESM7_ESM.mov
Supplementary Movie 2. Slow burst of a necrotic cell. MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 min for 12 h. Propidium iodide (red) labels permeable dying cells. (MOV 2427 kb)
10495_2011_587_MOESM8_ESM.mov
Supplementary Movie 3. Rapid burst of another necrotic cell. MDCK monolayers incubated with the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 min for 12 h. Propidium iodide (red) labels permeable dying cells. (MOV 833 kb)
Rights and permissions
About this article
Cite this article
Andrade, D., Rosenblatt, J. Apoptotic regulation of epithelial cellular extrusion. Apoptosis 16, 491–501 (2011). https://doi.org/10.1007/s10495-011-0587-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-011-0587-z