Abstract
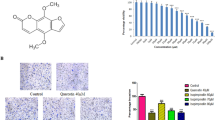
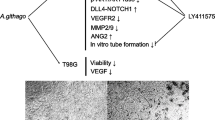
The major active constituents of ginseng are ginsenosides, and Rg1 is a predominant compound of the total extract. Recent studies have demonstrated that Rg1 can promote angiogenesis in vivo and in vitro. In this study, we used a DNA microarray technology to elucidate the mechanisms of action of Rg1. We report that Rg1 induces the proliferation of HUVECs, monitored using [3H]-thymidine incorporation and Trypan blue exclusion assays. Furthermore, Rg1 (150–600 nM) also showed an enhanced tube forming inducing effect on the HUVEC. Rg1 was also demonstrated to promote angiogenesis in an in vivo Matrigel plug assay, and increase endothelial sprouting in the ex vivo rat aorta ring assay. Differential gene expression profile of HUVEC following treatment with Rg1 revealed the expression of genes related to cell adhesion, migration and cytoskeleton, including RhoA, RhoB, IQGAP1, CALM2, Vav2 and LAMA4. Our results suggest that Rg1 can promote angiogenesis in multiple models, and this effect is partly due to the modulation of genes that are involved in the cytoskeletal dynamics, cell–cell adhesion and migration.
Similar content being viewed by others
References
1. Liu CX, Xiao PG. (1992). Recent advances on ginseng research in China. J Ethnopharmacol 36:27–38
2. Wang BX, Cui JC, Liu AJ, Wu SK. (1983). Studies on the anti-fatigue effect of saponins of stems and leaves of panax ginseng (SSLG). J Tradit Chin Med 3:89–94
3. Takahashi M, Tokuyama S, Kaneto H. (1992). Anti-stress effect of ginseng on the inhibition of the development of morphine tolerance in stressed mice. Jpn J Pharmacol 59:399–404
4. Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. (2002). Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 51:1851–1858
5. Gill CN. (1997). Panax ginseng pharmacology: a nitric oxide link. Biochem Pharmacol 54:1–8
6. Folkman J, Yuen S. (1992). Angiogenesis. J Biol Chem 267:10931–10934
7. Tonnesen MG, Feng X, Clark RA. (2000). Angiogenesis in wound healing. J Investig Dermatol Symp Proc 5:40–4
8. Favier J, Corvol P. (2001). Physiological angiogenesis. Therapie 56:455–463
9. Folkman J. (1974). Tumor angiogenesis. Adv Cancer Res 19:331–358
10. Walsh DA. (1999). Angiogenesis and arthritis. Rheumatology 38:103–112
11. Paleolog EM. (2002). Angiogenesis in rheumatoid arthritis. Arthritis Res 3:S81–S90
12. Funstsu H, Yamasshita H, Noma H, Shimizu E, Yamashita T, Hori S. (2001). Stimulation and inhibition of angiogenesis in diabetic retinopathy. Jpn J Ophthalmol 45, 577–584
13. Morisaki N, Watanabe S, Tezuka M, Zenibayashi M, Shiina R, Koyama N, Kanzaki T, Saito Y. (1995). Mechanism of angiogenic effects of saponin from ginseng Radix rubra in human umbilical vein endothelial cells. Br J Pharmacol 115:1188–1193
14. Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. (1994). Inhibitory of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull 17:635–639
15. Tao H, Yao M, Zou S, Zhao D, Qiu H. (2002). Effect of angiogenesis inhibitor Rg3 on the growth and metastasis of gastric cancer in SCID mice. Zhounghua Wai Ke Za Zhi 40:606–608
16. Shiladitya S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RNS, Sassisekharan R, Fan TPD. (2004). Modulating angiogenesis. The Yin and the Yang in Ginseng. Circulation 110:1219–1225
17. Passaniti A, Taylor RM, Pili R, Yue G, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. (1992). Methods in laboratory investigation. Lab Invest 67:519–528
18. Nicosia RF, Ottinetti A. (1990). Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol 26:119–128
19. Nicosia RF, Ottinetti A. (1990). Growth of microvessels in serum-free matrix culture of aorta. Lab Invest 63:115–122
20. Hegde P, Qi R, Abernathy K, Gay C, Dharap S, Gaspard R, Hughes JE, Snesrud E, Lee N, Quackenbush J. (2000). Concise guide to cDNA microarray analysis. Biotechnique 29:548–562
21. Tabuchi Y, Kondo T, Ogawa R, Mori H. (2002). DNA microarray analysis of genes elicited by ultrasound in human U937 cells. Biochem Biophys Res Commun 290:498–503
22. Isner JM, Asahara T. (1998). Therapeutic angiogenesis. Front Biosci 3:e49–69
23. Risau W. (1997). Mechanisms of angiogenesis. Nature 386:671–674
24. Shinkai K, Akedo H, Mukai M, Imamura F, Isoai A, Kobayashi M, Kitagawa I. (1996). Inhibitory of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res 87:357–362
25. Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. (1997). Ginsenoside-Rg1, one of the major active moleculaes from panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol 133:135–140
26. Chung E, Lee KY, Lee YJ, Lee YH, Lee SK. (1998). Ginsenoside-Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroid 63:421–424
27. Newton R. (2000). Molecular mechanisms of glucocorticoid action: what is important?. Thorax 55:603–613
28. Cardena GG, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. (1998). Dynamic activation of endothelial nitric oxide synthease by HSP90. Nature 392:821–824
29. Braga VM, Machesky LM, Hall A, Hotchin NA. (1997). The small GTPase Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 137:1421–1431
30. Fukata M, Nakagawa M, Kuroda S, Kaibuchi K. (1999). Commentary Cell adhesion amd Rho small GTPase. J Cell Sci 112:4491–4500
31. Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. (1994). Rac p21 is involved in insulin-induced memebrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol 14:2447–2456
32. Ridley AJ, Hall A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399
33. Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. (1994). Involvement of Rho p21 samll GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene 9:273–279
34. Schmitz AAP, Govek EE, Bottner B, Aelst LV. (2000). Rho GTPases: signaling, migration, and invasion. Exp Cell Res 261:1–12
35. Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. (1997). Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol 139:1047–1059
36. Braga VM, Del MA, Machesky L, Dejana E. (1999). Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell 10:9–22
37. Kaibuchi K, Kuroda S, Amano M. (1999). Regukation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 68:459–486
38. Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. (1999). Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol 11:591–596
39. Braga V. (2000). Epithelial cell shape: cadherins and small GTPases. Exp Cell Res 261:83–90
40. Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. (2000). Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem 275:10141–10149
41. Bustelo XR. (2000). Regulatory and signaling properties of the Vav family. Mol Cell Biol 20:1461–1477
42. Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. (1997). Phosphototyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385:169–172
43. Liu BP, Burridge K. (2000). Vav2 activates rac1, Cdc42, and RhoA downstream from growth factor receptor but not β1 integrins. Mol Cell Biol 20:7160–7169
44. Aelst LV, Schorey CD. (1997). Rho GTPases and signaling networks. Genes Dev 11:2295–2322
45. Noren NK, Liu BP, Burridge K, Kreft B. (2000). P120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 150:567–579
46. Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T. (1998). Role of IQGAP1, a target of small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science 281:832–835
47. Kuroda S, Fukata M, Nakagawa M, Kaibuchi K. (1999). Breakthroughs and views Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun 262:1–6
48. Fukata M, Kuroda S, Fujii K, Nakamurat T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. (1997). Regulation of cross-linking of actin filament by IQGAP1 a target for Cdc42. J Biol Chem 272:29579–29583
49. Joyal JL, Roland SA, Ho YD, Huddleston ME, Carr SA, Hart MJ, Scaks DB. (1997). Calmodulin modulates the interaction between IQGAP1 and Cdc42. J Biol Chem 272:15419–15425
50. Ho YD, Joyal JL, Li ZG, Scaks DB. (1999). IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem 274, 464–470
51. Li ZG, Kim SH, Higgin JMG, Brennert MB, Sacks DB. (1999). IQGAP1 and calmodulin modulate E-cadherin function. J Biol Chem 274:37885–37892
52. Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, Smet PD, Travers M, Tovry SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. (2001). Calcium signaling – an overview. Cell Dev Biol 12:3–10
53. Tashiro K, Sephel GC, Weeks BSM, Martin GR, Kleinman HK, Yamada Y. (1989). A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem 264:16174–16182
54. Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS. (1993). The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm 47:161–186
55. Grant DS, Tashiro K, Segui RB, Yamada Y, Martin GR, Kleinman HK (1989). Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structure in vitro. Cell 58:933–943
56. Malinda KM, Nomizu M, Chung M, Delgado M, Kiuratomi Y, Yamada Y, Kleinman HK. (1999). Identification of laminin α1 and β1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J 13:53–62
57. Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JCR. (2000). Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell 12:85–100
58. Greif H, Ben CJ, Shimon T, Bechor F, Eldar H, Livneh E. (1992). The protein kinase C-related PKC-L (eta) gene product is localized in the cell nucleus. Mol Cell Biol 12:1304–1311
59. Verkman AS, Hoek ANV, Ma T, Frigeri A, Skach WR, Mitra A, Tamarappoo BK, Farinas J. (1996). Water transport across mammalian cell membranes. Am J Physiol 270:C12–C30
60. Borgnia M, Nielsen S, Engel A, Agre P. (1999). Cellular and molecular biology and the aquaporin water channels. Annu Rev Biochem 68:428–458
61. Verkman AS, Mitra AK. (2000). Structure and function of aquaporin water channels. Am J Physiol Renal Physiol 278:F13–F28
62. Matsuzaki T, Tajika Y, Tserentsoodol N, Suzuki T, Hagiwara H, Takata K. (2002). Aquaporins: a water channel family. Anat Sci Int 77:85–93
63. Verkman AS. (2002). Aquaporin water channels and endothelial cell function. J Anat 200:617–627
64. Banthorpe DV. (1994). Terpenoids. In: Mann J (eds). Natural Products. Longman Scientific and technical, Essex, pp. 331–339
Acknowledgements
W wish to thank Dr. Shiladitya Sengupta, Biological Engineering Division, Massachusetts Institute of Technology, Cambridge, United Kingdom, for the valuable comments on this manuscript. We also thank Mr. Micheal Tsang and Miss. Emily Leung for their help in the proliferation assay. We are very grateful for the technical assistance in histological staining of Mr. Victor Ma and Mr. Cadmon Lim, Department of Clinical Oncology, Queen Elizabeth Hospital, HK. We also thank Mr. Kevin Kok and Miss. Fiona Luong for their help on Matrigel plug assay. We thank Dr. Simon Lee, Department of biochemistry, Chinese University of Hong Kong, for sharing experience on microarray data analysis. This work was supported by the Earmark Research grants (HKBU 2001/99M, HKBU 2171/03M) of the Research Grant Committee, Hong Kong SAR Government; Faculty Research Grant of the Hong Kong Baptist University (FRG/01-02/I-05).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10456-006-9036-y
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yue, P.Y., Wong, D.Y., Ha, W. et al. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg1 in vivo and in vitro . Angiogenesis 8, 205–216 (2005). https://doi.org/10.1007/s10456-005-9000-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-005-9000-2