Abstract
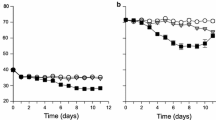
Amoebae grazing can be an important loss factor for blooms of the common cyanobacterium Microcystis. Some Microcystis strains seem to be protected against amoebae grazing, but it is unclear whether this is achieved by their colony morphology or biochemically. These factors were investigated in grazing experiments using two Microcystis-grazing amoebae (Korotnevella sp. and Vannella sp.) and two Microcystis strains with differing colony morphology (aeruginosa and viridis morphotype) and different sensitivity to amoebae grazing. Amoebae did not increase in density and failed to reduce the growth rate of cultures of the amoebae insensitive viridis strain, irrespective of whether the Microcystis strain was colonial or unicellular. This suggests that the extended mucilage matrix surrounding viridis colonies is not the main defence mechanism against amoebae grazing. At the same time, the growth rate of both unicellular and colonial cultures of the amoebae-sensitive aeruginosa strain was heavily reduced by the growing amoebae. The addition of filtered viridis-conditioned medium to aeruginosa cultures significantly decreased both amoebae growth and its effect on aeruginosa growth rates, which indicates that extracellular compounds constitutively produced by viridis are at least partially responsible for their insensitivity to amoebae grazing. These results demonstrate the potential importance of chemical interactions between lower trophic levels (protists) for Microcystis bloom dynamics.





Similar content being viewed by others
References
Allen PG, Dawidowicz EA (1990) Phagocytosis in Acanthamoeba 1 A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol 145(3):508–513
Anderson OR, Rogerson A (1995) Annual abundances and growth-potential of Gymnamoebae in the Hudson Estuary with comparative data from the Firth of Clyde. Eur J Protistol 31(2):223–233
Becker S, Matthijs HCP, Van Donk E (2010) Biotic factors in induced defence revisited: cell aggregate formation in the toxic cyanobacterium Microcystis aeruginosa PCC 7806 is triggered by spent Daphnia medium and disrupted cells. Hydrobiologia 644(1):159–168
Berry JP, Gantar M, Perez MH, Berry G, Noriega FG (2008) Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar Drugs 6(2):117–146
Brautigan DL (1995) Flicking the switches - phosphorylation of serine/threonine protein phosphatases. Semin Cancer Biol 6(4):211–217
Brunberg AK (1999) Contribution of bacteria in the mucilage of Microcystis spp (Cyanobacteria) to benthic and pelagic bacterial production in a hypereutrophic lake. Fems Microbiol Ecol 29(1):13–22
Camacho FA, Thacker RW (2006) Amphipod herbivory on the freshwater cyanobacterium Lyngbya wollei: Chemical stimulants and morphological defenses. Limnol Oceanogr 51(4):1870–1875
Czarnecki O, Henning M, Lippert I, Welker M (2006) Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environ Microbiol 8(1):77–87
Dao TS, Do-Hong LC, Wiegand C (2010) Chronic effects of cyanobacterial toxins on Daphnia magna and their offspring. Toxicon 55(7):1244–1254
Declerck P, Behets J, de Keersmaecker B, Ollevier F (2007) Receptor-mediated uptake of Legionella pneumophila by Acanthamoeba castellanii and Naegleria lovaniensis. J Appl Microbiol 103(6):2697–2703
Demott WR (1999) Foraging strategies and growth inhibition in five daphnids feeding on mixtures of a toxic cyanobacterium and a green alga. Freshw Biol 42(2):263–274
Ferrao AD, Da Costa SM, Ribeiro MGL, Azevedo S (2008) Effects of a saxitoxin-producer strain of Cylindrospermopsis raciborskii (Cyanobacteria) on the swimming movements of cladocerans. Environ Toxicol 23(2):161–168
Forni C, Telo FR, Caiola MG (1997) Comparative analysis of the polysaccharides produced by different species of Microcystis (Chroococcales, Cyanophyta). Phycologia 36(3):181–185
Fulton RS, Paerl HW (1987) Toxic and inhibitory effects of the blue-green-alga Microcystis-aeruginosa on herbivorous zooplankton. J Plankton Res 9:837–855
Fyda J, Fialkowska E, Pajdak-Stos A (2010) Dynamics of cyanobacteria-ciliate grazer activity in bitrophic and tritrophic microcosms. Aquat Microb Ecol 59(1):45–53
Gademann K, Portmann C (2008) Secondary metabolites from cyanobacteria: complex structures and powerful bioactivities. Curr Org Chem 12(4):326–341
Guillard RRL, Lorenzen CL (1972) Yellow-green algae with chlorophyllide C. J Phycol 8:10–14
Ha K, Jang MH, Takamura N (2004) Colony formation in planktonic algae induced by zooplankton culture media filtrate. J Freshwater Ecol 19(1):9–16
Hansson LA, Gustafsson S, Rengefors K, Bomark L (2007) Cyanobacterial chemical warfare affects zooplankton community composition. Freshw Biol 52(7):1290–1301
Ianora A, Miralto A, Poulet SA, Carotenuto Y, Buttino I, Romano G, Casotti R, Pohnert G, Wichard T, Colucci-D’amato L, Terrazzano G, Smetacek V (2004) Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 429(6990):403–407
Joung SH, Kim CJ, ahn CY, Jang KY, Boo SM, Oh HM (2006) Simple method for a cell count of the colonial cyanobacterium, Microcystis sp. J Microbiol 44(5):562–565
Jungmann D (1995) Isolation, purification, and characterization of new Daphnia-toxic compound from axenic Microcystis flos-aquae strain PCC7806. J Chem Ecol 21(11):1665–1676
Kehr JC, Zilliges Y, Springer A, Disney MD, Ratner DD, Bouchier C, Seeberger PH, Tandeau de Marsac N, Dittmann E (2006) A mannan binding lectin is involved in cell–cell attachment in a toxic strain of Microcystis aeruginosa. Mol Microbiol 59:893–906
Kim BR, Nakan S, Kim BH, Han MS (2006) Grazing and growth of the heterotrophic flagellate Diphylleia rotans on the cyanobacterium Microcystis aeruginosa. Aquat Microb Ecol 45(2):163–170
Komárková J, Šimek K (2003) Unicellular and colonial formations of picoplanktonic cyanobacteria under variable environmental conditions and predation pressure. Arch Hydrobiol Alg Stud 109:327–340
Kuhlmann HW, Kusch J, Heckmann K (1999) Predator induced defenses in ciliated protozoa. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, New Jersey, pp 142–155
Lampert W (1987) Laboratory studies on zooplankton-cyanobacteria interactions. New Zeal J Mar Fresh 21:483–490
Lampert W, Tollrian R, Stibor H (1994) Chemical induction of defense-mechanisms in fresh-water animals. Naturwissenschaften 81(9):375–382
Luo W, Pflugmacher F, Proschold T, Walz N, Krienitz L (2006) Genotype versus phenotype variability in Chlorella and Micractinium (Chlorophyta, Trebouxiophyceae). Protist 157(3):315–333
Lürling M, van Donk E (1996) Zooplankton-induced unicell-colony transformation in Scenedesmus acutus and its effects on growth of herbivore Daphnia. Oecologia 108(3):432–437
Maruyama T, Kato K, Yokoyama A, Tanaka T, Hiraishi A, Park HD (2003) Dynamics of microcystin-degrading bacteria in mucilage of Microcystis. Microbial Ecol 46(2):279–288
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45(3):569–579
Nishibe Y, Kawabata Z, Nakano S (2002) Grazing on Microcystis aeruginosa by the heterotrophic flagellate Collodictyon triciliatum in a hypertrophic pond. Aquat Microb Ecol 29(2):173–179
Nishibe Y, Manage PM, Kawabata Z, Nakano S (2004) Trophic coupling of a testate amoeba and Microcystis species in a hypertrophic pond. Limnology 5(2):71–76
Otsuka S, Suda S, Shibata S, Oyaizu H, Matsumoto S, Watanabe MM (2001) A proposal for the unification of five species of the cyanobacterial genus Microcystis Kutzing ex Lemmermann 1907 under the rules of the Bacteriological Code. Int J Syst Evol Micr 51:873–879
Rengefors K, Karlsson I, Hansson L-A (1998) Algal cyst dormancy: a temporal escape from herbivory. Proc R Soc Lond B 265:1353–1358
Reynolds CS (2007) Variability in the provision and function of mucilage in phytoplankton: facultative responses to the environment. Hydrobiologia 578(1):37–45
Rogerson A, Gwaltney C (2000) High numbers of naked amoebae in the planktonic waters of a mangrove stand in southern Florida, USA. J Eukaryot Microbiol 47(3):235–241
Rogerson A, Anderson OR, Vogel C (2003) Are planktonic naked amoebae predominately floc associated or free in the water column? J Plankton Res 25(11):1359–1365
Rohrlack T, Hyenstrand P (2007) Fate of intracellular microcystins in the cyanobacterium Microcystis aeruginosa (Chroococcales, Cyanophyceae). Phycologia 46(3):277–283
Rohrlack T, Henning M, Kohl JG (1999) Mechanisms of the inhibitory effect of the cyanobacterium Microcystis aeruginosa on Daphnia galeata’s ingestion rate. J Plankton Res 21(8):1489–1500
Rohrlack T, Dittmann E, Borner T, Christoffersen K (2001) Effects of cell-bound microcystins on survival and feeding of Daphnia spp. Appl Environ Microb 67(8):3523–3529
Rohrlack T, Christoffersen K, Hansen PE, Zhang W, Czarnecki O, Henning M, Fastner J, Erhard M, Neilan BA, Kaebernick M (2003) Isolation, characterization, and quantitative analysis of microviridin J, a new Microcystis metabolite toxic to Daphnia. J Chem Ecol 29(8):1757–1770
Sarnelle O, Gustafsson S, Hansson LA (2010) Effects of cyanobacteria on fitness components of the herbivore Daphnia. J Plankton Res 32(4):471–477
Selander E, Thor P, Toth G, Pavia H (2006) Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc R Soc B 273(1594):1673–1680
Shi LM, Cai YF, Yang HL, Xing P, Li PF, Kong LD, Kong FX (2009) Phylogenetic diversity and specificity of bacteria associated with Microcystis aeruginosa and other cyanobacteria. J Environ Sci-China 21(11):1581–1590
Sivonen K, Jones G (1999) Cyanobacterial Toxins. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. E & FN Spon, London, pp 41–111
Sønstebø JH, Rohrlack T (2011) Possible implications of chytrid parasitism for population subdivision in freshwater cyanobacteria of the genus Planktothrix. Appl Environ Microb 77(4):1344–1351
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Van Donk E, Cerbin S, Wilken S, Helmsing NR, Ptacnik R, Verschoor AM (2009) The effect of a mixotrophic chrysophyte on toxic and colony-forming cyanobacteria. Freshw Biol 54(9):1843–1855
van Gremberghe I, Vanormelingen P, Vanelslander B, Van der Gucht K, D’Hondt S, De Meester L, Vyverman W (2009) Genotype-dependent interactions among sympatric Microcystis strains mediated by Daphnia grazing. Oikos 118(11):1647–1658
van Gremberghe I, Leliaert F, Mergeay J, Vanormelingen P, Van der Gucht K, Debeer A-E, Lacerot G, De Meester L, Vyverman W (2011) Lack of phylogeographic structure in the freshwater cyanobacterium Microcystis aeruginosa indicates global dispersal and true cosmopolitanism. Plos One 6:e19561
Van Wichelen J, van Gremberghe I, Vanormelingen P, Debeer A-E, Leporcq B, Menzel D, Codd GA, Descy JP, Vyverman W (2010) Strong effects of amoebae grazing on the biomass and genetic structure of a Microcystis bloom (Cyanobacteria). Environ Microbiol 12(10):2797–2813
Verschoor AM, van der Stap I, Helmsing NR, Lurling M, van Donk E (2004) Inducible colony formation within the Scenedesmaceae: Adaptive responses to infochemicals from two different herbivore taxa. J Phycol 40(5):808–814
Waite AM, Olson RJ, Dan HG, Passow U (1995) Sugar-containing compounds on the cell surfaces of marine diatoms measured using concanavalin A and flow cytometry. J Phycol 31(6):925–933
Welker M, Sejnohova L, Nemethova D, von Dohren H, Jarkovsky J, Marsalek B (2007) Seasonal shifts in chemotype composition of Microcystis sp communities in the pelagial and the sediment of a shallow reservoir. Limnol Oceanogr 52(2):609–619
Wilken S, Wiezer S, Huisman J, Van Donk E (2010) Microcystins do not provide anti-herbivore defense against mixotrophic flagellates. Aquat Microb Ecol 59(3):207–216
Yang Z, Kong FX, Cao HS, Shi XL (2005) Observation on colony formation of Microcystis aeruginosa induced by filtered lake water under laboratory conditions. Ann Limnol-Int J Lim 41(3):169–173
Yang Z, Kong FX, Shi XL, Cao HS (2006) Morphological response of Microcystis aeruginosa to grazing by different sorts of zooplankton. Hydrobiologia 563:225–230
Yang Z, Kong FX, Shi XL, Zhang M, Xing P, Cao HS (2008) Changes in the morphology and polysaccharide content of Microcystis aeruginosa (Cyanobacteria) during flagellate grazing. J Phycol 44(3):716–720
Yang Z, Kong FX, Zhang M, Yu Y, Qian SQ (2009) Effect of filtered cultures of flagellate Ochromonas sp on colony formation in Microcystis aeruginosa. Int Rev Hydrobiol 94(2):143–152
Zhang XM, Watanabe MM (2001) Grazing and growth of the mixotrophic chrysomonad Poterioochromonas malhamensis (Chrysophyceae) feeding on algae. J Phycol 37(5):738–743
Zhang M, Kong FX, Tan X, Yang Z, Cao HS, Xing P (2007) Biochemical, morphological, and genetic variations in Microcystis aeruginosa due to colony disaggregation. World J Microb Biot 23(5):663–670
Zhang X, Hu HY, Hong Y, Yang J (2008) Isolation of a Poterioochromonas capable of feeding on Microcystis aeruginosa and degrading microcystin-LR. FEMS Microbiol Lett 288(2):241–246
Acknowledgments
We sincerely thank Stina Drakare from the Swedish University of Agricultural Sciences for providing us with Microcystis bloom samples from Lake Mälaren in Sweden and Karen Soenen, Lancelot Blondeel and Ahmed Abdul Jabbar for technical assistance during the experiments. Two anonymous reviewers gave valuable and highly appreciated comments that have lead to a considerable improvement of this paper. P.V. is a postdoctoral research fellow with the Research Foundation – Flanders (FWO). This research was financially supported by the BELSPO (Belgian Science Policy) project B-BLOOMS2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Rights and permissions
About this article
Cite this article
Van Wichelen, J., van Gremberghe, I., Vanormelingen, P. et al. The importance of morphological versus chemical defences for the bloom-forming cyanobacterium Microcystis against amoebae grazing. Aquat Ecol 46, 73–84 (2012). https://doi.org/10.1007/s10452-011-9382-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-011-9382-8