Abstract
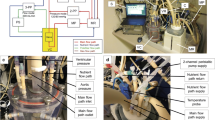
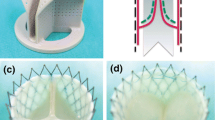
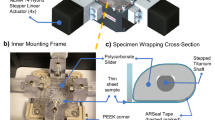
Current mechanical conditioning approaches for heart valve tissue engineering concentrate on mimicking the opening and closing behavior of the leaflets, either or not in combination with tissue straining. This study describes a novel approach by mimicking only the diastolic phase of the cardiac cycle, resulting in tissue straining. A novel, yet simplified, bioreactor system was developed for this purpose by applying a dynamic pressure difference over a closed tissue engineered valve, thereby inducing dynamic strains within the leaflets. Besides the use of dynamic strains, the developing leaflet tissues were exposed to prestrain induced by the use of a stented geometry. To demonstrate the feasibility of this strain-based conditioning approach, human heart valve leaflets were engineered and their mechanial behavior evaluated. The actual dynamic strain magnitude in the leaflets over time was estimated using numerical analyses. Preliminary results showed superior tissue formation and non-linear tissue-like mechanical properties in the strained valves when compared to non-loaded tissue strips. In conclusion, the strain-based conditioning approach, using both prestrain and dynamic strains, offers new possibilities for bioreactor design and optimization of tissue properties towards a tissue-engineered aortic human heart valve replacement.
Similar content being viewed by others
References
Barron, V., E. Lyons, C. Stenson-Cox, P. E. McHugh, and A. Pandit. Bioreactors for cardiovascular cell and tissue growth: A review. Ann. Biomed. Eng. 31:1017–1030, 2003.
Chapman, G. B., W. Durante, J. D. Hellums, and A. I. Schafer. Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 278:H748–H754, 2000.
Dethlefsen, S. M., D. Shepro, and P. A. D'Amore. Comparison of the effects of mechanical stimulation on venous and arterial smooth muscle cells in vivo. J. Vasc. Res. 33:405–413, 1996.
Driessen, N. J. B., C. V. C. Bouten, and F. P. T. Baaijens. A structural constitutive model for collagenous cardiovascular tissue incorporating the angular fiber distribution. J. Biomech. Eng. 127:494–503, 2005.
Dumont, K., J. Yperman, E. Verbeken, P. Segers, B. Meuris, S. Vandenberghe, W. Flameng, and P. R. Verdonck. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif. Organs 26:710–714, 2002.
Engelmayr, G. C., E. Rabkin, F. W. H. Sutherland, F. J. Schoen, J. E. Mayer, and M. S. Sacks. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials 26:175–187, 2005.
Hildebrand, D. K., Z. J. Wu, J. E. Mayer, and M. S. Sacks. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann. Biomed. Eng. 32:1039–1049, 2004.
Hoerstrup, S. P., R. Sodian, S. Daebritz, J. Wang, E. A. Bacha, D. P. Martin, A. M. Moran, K. J. Guleserian, J. S. Sperling, S. Kaushal, J. P. Vacanti, F. J. Schoen, and J. E. Mayer. Functional living trileaflet heart valves grown in vitro. Circulation 102(suppl III):III-44–III-49, 2000.
Hoerstrup, S. P., R. Sodian, J. S. Sperling, J. P. Vacanti, and J. E. Mayer. New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue Eng. 6:75–79, 2000.
Jockenhoevel, S., G. Zund, S. P. Hoerstrup, A. Schnell, and M. Turina. Cardiovascular tissue engineering: A new laminar flow chamber for in vitro improvement of mechanical tissue properties ASAIO J. 48:8–11, 2002.
Kim, B., J. Nikolovski, J. Bonadio, and D. J. Mooney. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat. Biotechnol. 17:979–983, 1999.
Kim, B., and D. J. Mooney. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J. Biomech. Eng. 122:210–215, 2000.
Lee, A. A., D. A. Graham, S. Dela Cruz, A. Ratcliffe, and W. J. Karlon. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J. Biomech. Eng. 124:37–43, 2002.
McCulloch, A. D., A. B. Harris, C. E. Sarraf, and M. Eastwood. New multi-cue bioreactor for tissue engineering of tubular cardiovascular samples under physiological conditions. Tissue Eng. 10:565–573, 2004.
Mironov, V., V. Kasyanov, K. McAllister, S. Oliver, J. Sistino, and R. Markwald. Perfusion bioreactor for vascular tissue engineering with capacities for longitudinal stretch. J. Cranio-fac. Surg. 14:340–347, 2003.
Mol, A., C. V. C. Bouten, G. Zund, C. I. Guenter, J. F. Visjager, M. I. Turina, F. P. T. Baaijens, and S. P. Hoerstrup. The relevance of large strains in functional tissue engineering of heart valves. Thorac. Cardiovasc. Surg. 51:78–83, 2003.
Mol, A., M. I. van Lieshout, G. C. Dam-de Veen, S. Neuenschwander, S. P. Hoerstrup, F. P. T. Baaijens, and C. V. C. Bouten. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials 26:3113–3121, 2005.
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55–63, 1983.
Narita, Y., K. Hata, H. Kagami, A. Usui, M. Ueda, and Y. Ueda. Novel pulse duplicating bioreactor system for tissue-engineered vascular constructs. Tissue Eng. 10:1224–1233, 2004.
Niklason, L. E., J. Gao, W. M. Abbott, K. K. Hirschi, S. Houser, R. Marini, and R. Langer. Functional arteries grown in vitro. Science 284:489–493, 1999.
Niklason, L. E., W. Abott, J. Gao, B. Klagges, K. K. Hirschi, K. Ulubayram, N. Conroy, R. Jones, A. Vasanawala, S. Sanzgiri, and R. Langer. Morphological and mechanical characteristics of engineered bovine arteries. J. Vasc. Surg. 33:628–638, 2001.
O'Callaghan, C. J., and B. Williams. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells. Hypertension 36:319–324, 2000.
Rutten, M. C. M., M. Wijlaars, A. Mol, E. A. van Dam, G. J. Strijkers, K. Nicolay, and F. P. T. Baaijens. The valve exerciser: A novel bioreactor for physiological loading of tissue-engineered aortic valves. J. Biomech. (under revision).
Schned, A. R., K. J. Wheeler, C. A. Hodorowski, J. A. Heaney, M. S. Ernstoff, R. J. Amdur, and R. D. Harris. Tissue-shrinkage correction factor in the calculation of prostate cancer volumer. Am. J. Surg. Pathol 20:1501–1506, 1996.
Schnell, A. M., S. P. Hoerstrup, G. Zund, S. Kolb, R. Sodian, J. F. Visjager, J. Grunenfelder, A. Suter, and M. Turina. Optimal cell source for cardiovascular tissue engineering: Venous vs. aortic human myofibroblasts. Thorac. Cardiovasc. Surg. 49:221–225, 2001.
Segal, A. SEPRAN User Manual, Standard Problems and Programmers Guide. Leidschendam: Ingenieursbureau SEPRA, the Netherlands, 1984.
Seliktar, D., R. A. Black, R. P. Vito, and R. M. Nerem. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann. Biomed. Eng. 28:351–362, 2000.
Sodian, R., T. Lemke, C. Fritsche, S. P. Hoerstrup, P. Fu, E. V. Potapov, H. Hausmann, and R. Hetzer. Tissue-engineering bioreactors: A new combined cell-seeding and perfusion system for vascular tissue engineering. Tissue Eng. 8:863–870, 2002.
Stradins, P., R. Lacis, I. Ozolanta, B. Purina, V. Ose, L. Feldmane, and V. Kasyanov. Comparison of biochemical and structural properties between human aortic and pulmonary valve. Eur. J. Cardiothorac. Surg. 26:634–639, 2004.
Thompson, C. A., P. Colon-Hernandez, I. Pomerantseva, B. D. MacNeil, B. Nasseri, J. P. Vacanti, and S. N. Oesterle. A novel pulsatile, laminar flow bioreactor for the development of tissue-engineered vascular structures. Tissue Eng. 8:1083–1088, 2002.
Thubrikar, M. The Aortic Valve. Boca Raton: CRC Press, 1990, 221 pp.
Ueba, H., M. Kawakami, and T. Yaginuma. Shear stress as an inhibitor of vascular smooth muscle cell proliferation. Arterioscler. Thromb. Vasc. Biol. 17:1512–1516, 1997.
Watase, M., M. A. Awolesi, J. Ricotta, and B. E. Sumpio. Effect of pressure on cultured smooth muscle cells. Life Sci. 61:987–996, 1997.
Williams, C., and T. M. Wick. Perfusion bioreactor for small diameter tissue-engineered arteries. Tissue Eng. 10:930–941, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mol, A., Driessen, N.J.B., Rutten, M.C.M. et al. Tissue Engineering of Human Heart Valve Leaflets: A Novel Bioreactor for a Strain-Based Conditioning Approach. Ann Biomed Eng 33, 1778–1788 (2005). https://doi.org/10.1007/s10439-005-8025-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-8025-4