Abstract
Background: Invasive breast cancer is a frequently diagnosed disease that now comes with an ever expanding array of therapeutic management options. We assessed the effects of 20 prognostic factors in a multivariate context.
Methods: We accrued clinical data for 156 consecutive patients with stage 1–3 primary invasive breast cancer who were diagnosed in 1989–1990 at the Henrietta Banting Breast Center, and followed to 1995. There is complete follow-up for 91% of patients (median follow-up of 4.9 years). The event of interest was distant recurrence (for distant disease-free survival, DFS). We used Cox and log-normal step-wise regression to assess the multivariate effects of the following factors on DFS: age, tumor size, nodal status, histology, tumor and nuclear grade, lymphovascular and perineural invasion (LVPI), ductal carcinoma-in-situ (DCIS) type, DCIS extent, DCIS at edge of tumor, ER and PgR, ERICA, adjuvant systemic therapy, ki67, S-phase, DNA index, neu oncogene, and pRb.
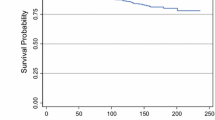
Results: There was strong evidence against the Cox assumption of proportional hazards for nodal status, and nodal status was not in the Cox step-wise model. With step-wise log-normal regression, a large tumor size (P < .001), positive nodes (P 5 .002), high nuclear grade (P 5 .01), presence of LVPI (P 5 .03), and infiltrating duct carcinoma not otherwise specified (P 5 .05) were associated with a reduction in DFS.
Conclusions: For nodal status, there was strong evidence against the Cox assumption of proportional hazards, and it was not included in the Cox model although it was in the log-normal model. Only traditional factors were included in the step-wise models. Thus, this statistical management of prognostic markers in breast cancer appears to be very important.
Similar content being viewed by others
References
Clark GM. 1992; Integrating prognostic factors. Breast Cancer Res Treat 22:187–91.
Chapman JW, Murray D, McCready DR, et al. 1996; An improved statistical approach: can it clarify the role of new prognostic factors for breast cancer? Euro J Cancer 32A:1949–56.
Chapman JW, Hanna W, Kahn HJ, Lickley HLA, Wall J, Fish EB, McCready DR. 1996; Alternative multivariate modelling for time to local recurrence for breast cancer patients receiving a lumpectomy alone. Surg Oncol 5:265–71.
Chapman JW, Trudeau ME, Pritchard KI, et al. 1992; A comparison of all subset Cox and accelerated failure time models with Cox step-wise regression for node positive breast cancer. Breast Cancer Res Treat 22:263–72.
Pritchard KI, Trudeau ME, Chapman JW, et al. 1993; Prognostic variables in node- negative breast cancer: an all subset analysis. Proc ASCO (Abstract) 12:68.
Hilsenbeck SG, Ravdin PM, de Moor CA, Osborne CK, Clark GM. 1996; Paradoxical decreases in prognostic utility as datasets mature: time-dependent lack of proportional hazards in prognostic factors in primary breast cancer. Breast Cancer Res Treat (Abstract) 37:35
Boag JW. 1949; Maximum likelihood estimates of the proportion of patients cured by cancer therapy. JRSS B 11:15–44.
Rutqvist LE, Wallgren A, Nilsson BO. 1984; Is breast Cancer a curable disease? Cancer 53:1793–800.
Gamel JW, Vogel RL, McLean IW. 1993; Assessing the impact of adjuvant therapy on cure rate for stage 2 breast carcinoma. Br J Cancer 68:115–8.
Gamel JW, Vogel RL. 1993; A model of long-term survival following adjuvant therapy for stage 2 breast cancer. Br J Cancer 68: 1167–70.
Gamel JW, Vogel RL, Valaguisa P, Bonadonna G. 1994; Parametric survival analysis of adjuvant therapy for stage II breast Cancer. Cancer 74:2483–490.
Gamel JW, Meyer JS, Province MA. 1995; Proliferative rate by S-phase measurement may affect cure of breast carcinoma. Cancer 76:1009–18.
Berg JW. 1965; The distribution of cancer deaths in time. A survey test of the lognormal model. Br J Cancer 19:695–711.
Kalbfleisch JD, Prentice RL. 1980 The Statistical Analysis of Failure Time Data. John Wiley and Sons, New York 21–35
Veronesi U, Marubini E, Del Vecchio M, et al. 1995; Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 87:19–27.
Sun J, Chapman JW, Gordon R, Sivaramakrishna R, Link MA, Fish EB. Survival from primary breast cancer by tumour size for the age groups with different mammography screening guidelines. Breast Cancer Res Treat (Abstract) 1998;50:281
Fan M, Chapman J, Link MA, Fish EB. 1999; Competing risks analysis for recurrence from primary breast cancer after lumpectomy. Breast Cancer Res Treat (Abstract) 57:41
Hallett D, Chapman JW, Gamel JW, McLean IW. 1999; Estimation of “cure” rate for intraocular melanoma. Proceed Am Assoc Cancer Res (Abstract) 40:43
Chapman JW, Wolman E, Wolman SR, et al. Assessing genetic markers of tumour progression in the context of intra-tumour heterogeneity. Cytometry 1998;31:67–73.
Chapman JW, Mobbs BG, McCready DR, et al. 1996; An investigation of cut-points for primary breast cancer estrogen and progesterone receptor assays. J Steroid Biochem Mol Biol 57:323–8.
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829 –35.
Gore SM, Pocock SJ, Kerr GR. 1984; Regression models and nonproportional hazards in the analysis of breast cancer survival. Appl Statist 33:176–95.
Allred DC, Harvey JM, Berardo M, Clark GM. 1998 Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–68.
Hayes DF, Bast RC, Desch CE, et al. 1996; Tumor marker utility grading system: a framework to evaluate clinical utility of tumour markers. J Natl Cancer Inst 88:1456–66.
Gusterson BA. 1995 The change in the role of the pathologist in the prediction of tumor behaviour and response to treatment. In: Dickson RB, Lippman HE eds. Doctors, Drugs and Hormonal Resistance in Breast Cancer. Ellis Harwood New York 39–53.
Locker AP, Ellis JO, Morgan DAL, Elston CW. 1989; Factors influencing local recurrence after excision and radiotherapy for primary breast cancer. Br J Surg 76:890–4
McCready DR, Hanna W, Kahn HJ, Chapman J, Wall J, Fish EB, Lickley HLA. Factors associated with local breast cancer recurrence after lumpectomy alone. Ann Surg Oncol 1996;3: 358–66.
Andrulis IL, Bull SB, Blackstein ME, et al. 1998; Neu/erbB-2 amplification identifies a poor- prognosis group of women with nodenegative breast cancer. J Clin Oncol 16:1340–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCready, D.R., Chapman, JA.W., Hanna, W.M. et al. Factors Affecting Distant Disease-Free Survival for Primary Invasive Breast Cancer: Use of a Log-Normal Survival Model. Ann Surg Oncol 7, 416–426 (2000). https://doi.org/10.1007/s10434-000-0416-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10434-000-0416-z