Abstract
Object
To construct an optimised, high-density receive array and a movement device to achieve dynamic imaging of the knee in orthopedic large animal models (e.g., minipigs) at 1.5 T.
Materials and methods
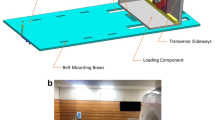
A 13-channel RF receive array was constructed, and the crucial choice of the array element size (based on considerations like region of interest, geometry of the minipig’s knee, achievable signal-to-noise ratio, applicability of parallel imaging, etc.) was determined using the Q factors of loops with different sizes. A special movement device was constructed to guide and produce a reproducible motion of the minipig’s knee during acquisition.
Results
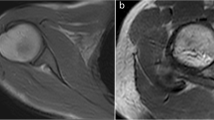
The constructed array was electrically characterised and the reproducibility of the cyclic motion was validated. Snapshots of dynamic in vivo images taken at a temporal resolution (308 ms) are presented. Some of the fine internal structures within the minipig’s knee, like cruciate ligaments, are traced in the snapshots.
Conclusion
This study is a step towards making dynamic imaging which can give additional information about joint injuries when static MRI is not able to give sufficient information, a routine clinical application. There, the combination of a high-density receive array and a movement device will be highly helpful in the diagnosis and therapy monitoring of knee injuries in the future.












Similar content being viewed by others
References
National Ambulatory Medical Care Survey 1998–2006. http://orthoinfo.aaos.org
Gammons M, Schwartz E (2011) ACL injury. http://emedicine.medscape.com/article/89442-overview Accessed 2 Nov 2011
Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS (2008) Function, osteoarthritis and activity after ACL rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc 16:442–448
Van Dijck RAHE, Saris DB, Willems JW, Fievez AWFM (2008) Additional surgery after anterior cruciate ligament reconstruction: can we improve technical aspects of the initial procedure? Fort Arthr 24(1):88–95
Chan D, Mohtadi NG, Dainty KN, Whelan DB (2008) Patellar tendon vs. hamstring autografts for primary ACL reconstruction: a cochrane review. J Bone Jt Surg Br 92-B:18
Liu H, Fan H, Wang Y, Toh SL, Goh JCH (2008) The interaction between combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials 29:662–674
Vavken P, Joshi S, Murray MM (2009) Triton-X is the most effective among three decellularization agents for ACL tissue engineering. J Orthop Res 27(12):1612–1618
Freeman JW, Woods MD, Cromer DA, Ekwueme EC, Andric T, Atiemo EA, Bijoux CH, Laurencin CT (2011) Evaluation of a hydrogel–fiber composite for ACL tissue engineering. J Biomech 44(4):694–699
Sahoo S, Ouyang H, Goh JCH, Tay TE, Toh SL (2006) Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng 12(1):91–99
Hwee TC (2007) Design and development of bioreactor for ligament tissue engineering. Master thesis, National University of Singapore
Brune T, Borel A, Gilbert TW, Franceschi JP, Badylak SF, Sommer P (2007) Invitro comparison of human fibroblasts from intact and ruptures ACL for use in tissue engineering. Eur Cells Mater 14:78–91
Virchenko O (2007) Stimulation of tendon repair by platelet concentrate, CDMP-2 and mechanical loading in animal models. Medical Dissertation no: 1005, Faculty of Health Sciences, Linköping university (Sweden)
Koh HL, Kshirsagar A, Herrod NJ, Carpenter TA, Hall LD, Hunziker EB, Tyler JA (1996) Visualisation by MRI of focal cartilage lesions in the excised mini-pig knee. J Orthop Res 14(4):554–561
Lee K-Y, Dunn TC, Steinbach LS, Ozhinsky E, Ries MD, Majumdar S (2004) Computer-aided quantification of focal cartilage lesions in osteoarthritic knee using MRI. Magn Reson Imaging 22(8):1105–1115
Petersen JP, Ueblacker P, Goepfert C, Adamietz P, Baumbach K, Stork A, Rueger JM, Poertner R, Amling M, Meenen NM (2008) Long term results after implantation of tissue engineered cartilage for the treatment of osteochondral lesions in a minipig model. J Mater Sci Mater Med 19:2029–2038
Baumbach K, Petersen JP, Ueblacker P, Schroeder J, Goepfert C, Stork A, Rueger JM, Amling M, Meenen NM (2008) The fate of osteochondral grafts after autologous osteochondral transplantation: a one-year follow-up study in a minipig model. Arch Orthop Trauma Surg 128:1255–1263
Jung M, Kaszap B, Redöhl A, Steck E, Breusch S, Richter W, Gotterbarm T (2009) Enhanced early tissue regeneration after matrix-assisted autologous mesenchymal stem cell transplantation in full thickness chondral defects in a minipig model. Cell Transpl 18:923–932
Allen AM, Horn AW (2011) MRI for anterior cruciate ligament injury. http://emedicine.medscape.com/article/400547-overview. Accessed 3 Aug 2011
Colombet P, Dejour D, Panisset JC, Siebold R, The French Arthroscopic Society (2010) Current concept of partial anterior cruciate ligament ruptures. Orthop Traumatal Surg Res 96:109–118
Draper CE, Santos JM, Kourtis LC, Besier TF, Fredericson M, Beaupre GS, Gold GE, Delp SL (2008) Feasibility of using real-time MRI to measure joint kinematics in 1.5 T and open-bore 0.5 T systems. J Magn Reson Imaging 28(1):158–166
Dragoo JL, Phillips C, Schmidt JD, Scanlan SF, Blazek K, Steadman JR, Williams A (2010) Mechanics of the anterior interval of the knee using open dynamic MRI. Clin Biomech 25:433–437
DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G (2006) The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sport Med 34(8):1240–1246
Johal P, Williams A, Wragg P, Hunt D, Gedroyc W (2005) Tibio-femoral movement in the living knee. A study of weight bearing and non-weight bearing knee kinematics using ‘interventional’ MRI. J Biomech 38:269–276
Patel VV, Hall K, Ries M, Lotz J, Ozhinsky E, Lindsey C, Lu Y, Majumdar S (2004) A three-dimensional MRI analysis of knee kinematics. J Orthop Res 22:283–292
Patel VV, Hall K, Ries M, Ozhinsky E, Lu Y, Majumdar S (2003) Magnetic resonance imaging of patellofemoral kinematics with weight bearing. J Bone Jt Surg 85A(12):2419–2424
Witonski D, Goraj B (1999) Patellar motion analyzed by kinematic and dynamic axial magnetic resonance imaging in patients with anterior knee pain syndrome. Arch Orthop Trauma Surg 119:46–49
Barrance PJ, Williams GN, Snyder-Mackler L, Buchanan TS (2005) Altered knee kinematics in ACL-deficient non-copers: a comparison using dynamic MRI. J Orthop Res 24(2):132–140
Sheehan FT, Zajac FE, Drace JE (1998) Using cine phase contrast magnetic resonance imaging to non-invasively study in vivo knee dynamics. J Biomech 31:21–26
Benjamin ma C, Lee K-Y, Schrumpf MA, Majumdar S (2005) Analysis of 3-dimensional in vivo knee kinematics using dynamic magnetic resonance imaging. Oper Tech Orthop 15:57–63
Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims J (2010) The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol 62:196–220
Zembsch A, Trattnig S, Walter J, Pölzl K-H, Ritschl P (1998) Positioning device for optimal active kinematic real-time magnetic resonance imaging of the knee joint: a technical note. Clin Biomech 13:308–313
Vedi V, Williams A, Tennant SJ, Spuse E, Hunt DM, Gedroyc WMW (1999) Meniscal movement: an in vivo study using dynamic MRI. J Bone Jt Surg [Br] 81-B:37–41
Haase A, Odoj F, Von Klielin M, Warnking J, Fidler F, Weisser A, Nittka M, Rommel E, Lanz T, Kalusche B, Griswold M (2000) NMR probeheads for in vivo applications. Concept Magnetic Res 12(6):361–388
Chen CN, Hoult DI (1985) Biomedical magnetic resonance technology. Institute of Physics Publishing, Bristol and Philadelphia
Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A (2002) Generalised auto-calibrating partially parallal acquisitions. Magn Reson Med 47:1202–1210
Pruesmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42:952–962
Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL (2009) 96-channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn Reson Med 62:754–762
Riffe MJ, Blaimer M, Barkauskas KJ, Duerk JL, Griswold MA (2007) SNR estimation in fast dynamic imaging using bootstrapped statistics. Proc Intl Soc Mag Reson Med 15:1879
Breuer FA, Kannengiesser SAR, Blaimer M, Sieberlich N, Jakob PM, Griswold PM (2009) General formulation for quantitative G-factor calculation in GRAPPA reconstructions. Magn Reson Med 62:739–746
Acknowledgments
Project ForZebra (www.forzebra.de), Dr. Andre Steinert & Dr. Alexander Nedopil, Orthopedic Center for Musculoskeletal Research, Keonig-Ludwig-Haus, University of Wuerzburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sairamesh Raghuraman and Joachim H. X. Schrauth contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Raghuraman, S., Schrauth, J.H.X., Weber, D.L. et al. Dynamic MR imaging of a minipig’s knee using a high-density multi-channel receive array and a movement device. Magn Reson Mater Phy 26, 215–228 (2013). https://doi.org/10.1007/s10334-012-0341-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-012-0341-8