Abstract
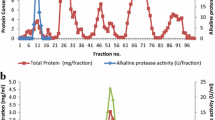
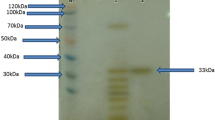
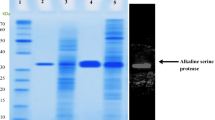
An alkaline protease secreting Haloalkaliphilic bacterium (Gene bank accession number EU118361) was isolated from the Saurashtra Coast in Western India. The alkaline protease was purified by a single step chromatography on phenyl sepharose 6 FF with 28% yield. The molecular mass was 40 kDa as judged by SDS-PAGE. The enzyme displayed catalysis and stability over pH 8–13, optimally at 9–11. It was stable with 0–4 M NaCl and required 150 mM NaCl for optimum catalysis at 37 °C; however, the salt requirement for optimal catalysis increased with temperature. While crude enzyme was active at 25–80 °C (optimum at 50 °C), the purified enzyme had temperature optimum at 37 °C, which shifted to 80 °C in the presence of 2 M NaCl. The NaCl not only shifted the temperature profile but also enhanced the substrate affinity of the enzyme as reflected by the increase in the catalytic constant (K cat). The enzyme was also calcium dependent and with 2 mM Ca+2, the activity reached to maximum at 50 °C. The crude enzyme was highly thermostable (37–90 °C); however, the purified enzyme lost its stability above 50 °C and its half life was enhanced by 30 and sevenfold at 60 °C with 1 M NaCl and 50 mM Ca+2, respectively. The activity of the enzyme was inhibited by PMSF, indicating its serine type. While the activity was slightly enhanced by Tween-80 (0.2%) and Triton X-100 (0.05%), it marginally decreased with SDS. In addition, the enzyme was highly stable with oxidizing-reducing agents and commercial detergents and was affected by metal ions to varying extent. The study assumes significance due to the enzyme stability under the dual extremities of pH and salt coupled with moderate thermal tolerance. Besides, the facts emerged on the enzyme stability would add to the limited information on this enzyme from Haloalkaliphilic bacteria.







Similar content being viewed by others
References
Adams RJ, Bygraves M, Kogut, Russell NJ (1987) The role of osmotic effects in haloadaptation of Vibrio costicola. J Gen Microbiol 133:1861–1870
Adinarayana K, Ellaiah P, Prasad DS (2003) Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmScitech 4:56
Bakhtiar S, Andersson M, Gessesse A, Mattiasson B, Kaul R (2002) Stability characteristics of a calcium-independent alkaline protease from Nesterenkonia sp. Enz Microbial Technol 32:525–531
Banerjee UC, Sani RK, Azmi W, Soni R (1999) Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem 35:213–219
Bayoudh A, Gharsallah N, Chamkha M, Dhouib A, Ammar S, Nasri M (2000) Purification and characterization of an alkaline protease from Pseudomonas aeruginosa MN1. J Ind Microbiol Biotechnol 24:291–295
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Catara G, Ruggiero G, La Cara F, Digilio FA, Capasso A, Rossi MA (2003) Novel extracellular subtilisin-like protease from the hyperthermophile Aeropyrum pernix K1: biochemical properties, cloning, and expression. Extremophiles 7:391–399
David R, Arahal M, Marquez C, Volcani BE, Schleifer KH, Ventosa A (1999) Bacillus marismortui sp. nov., a new moderately halophilic species from the Dead Sea. Int J Syst Evol Microbiol 49:521–530
Dodia MS, Joshi RH, Patel RK, Singh SP (2006) Characterization and stability of extracellular alkaline proteases from moderately halophilic and alkaliphilic Bacteria isolated from saline habitat of coastal Gujarat, India. Braz J Microbiol 37:276–282
Donaghy JA, McKay AM (1993) Production and properties of an alkaline protease by Aureobasidium pullulans. J Appl Bacteriol 74:662–666
Doronina N, Darmaeva T, Trotsenko Y (2003) Methylophaga natronica sp. nov., a new alkaliphilic and moderately halophilic, restricted-facultatively methylotrophic bacterium from soda lake of the Southern Transbaikal region. Syst Appl Microbiol 26:382–389
Eddy ML, Jablonski PE (2000) Purification and characterization of a membrane-associated ATPase from Natronococcus occultus, a haloalkaliphilic archaeon. FEMS Microbiol Lett 189:211–214
Elsztein C, Herrera SMK, Sanchez JJ, de Castro RE (2001) Autoproteolytic activation of the haloalkaliphilic archaeon Natronococcus occultus extracellular serine protease. J Basic Microbiol 41:319–27
Estell DA, Graycar TP, Wells JA (1985) Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J Biol Chem 260:6518–6521
Foti M, Ma S, Sorokin DY, Rademaker JL, Kuenen JG, Muyzer G (2006) Genetic diversity and biogeography of haloalkaliphilic sulphur-oxidizing bacteria belonging to the genus Thioalkalivibrio. FEMS Microbiol Ecol 56:95–101
Gessesse A, Kaul RH, Gashe BA, Mattiasson B (2003) Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enz Microbial Technol 32:519–524
Gimenez MI, Studdert CA, Sanchez J, De Castro RE (2000) Extracellular protease of Natrialba magadii: purification and biochemical characterization. Extremophiles 4:181–188
Goller K, Galinski EA (1999) Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J Mol Catl B Enzym 7:37–45
Gupta A, Roy I, Patel RK, Singh SP, Khare SK, Gupta MN (2005) A One-step purification and characterization of an alkaline protease from haloalkaliphilic Bacillus sp. Journal of Chromatogr A 1075:103–108
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Hagihara B (1958) The Enzymes, vol 4. Academic press Inc, New York
Herrera SK, Studdert C, Sanchez J, De Castro R (1997) Intracellular proteolytic activity of the haloalkaliphilic archaeon Natronococcus occultus. Effect of starvation. J Basic Microbiol 7:313–322
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulphate and copolymerized substrates. Anal Biochem 102:196–202
Hoover RB, Pikuta EV, Bej AK, Marsic D, Whitman WB, Tang J, Krader P (2003) Spirochaeta americana sp. nov., a new haloalkaliphilic, obligately anaerobic spirochaete isolated from soda Mono Lake in California. Int J Syst Evol Microbiol 53:815–821
Huang Q, Peng Y, Li X, Wang Y, Zhang Y (2003) Purification and characterization of an extracellular alkaline serine protease with dehairing function from Bacillus pumilus. Curr Microbiol 46:169
Izotova LS, Strongin AY, Chekulaeva LN, Sterkin VE, Ostoslavskaya VI, Lyublinskaya LA, Timokhina EA, Stepanov VM (1983) Purification and properties of serine protease from Halobacterium halobium. J Bacteriol 155:826–830
Jogi C, Joshi RH, Dodia MS, Singh SP (2005) Extracellular alkaline protease from haloalkaliphilic bacteria isolated from sea water along coastal Gujarat. J Cell Tissue Res 5:439–444
Kobayashi T, Kanai H, Hayashi T, Akiba T, Akaboshi R, Horikoshi K (1992) Haloalkaliphilic maltotriose-forming a-amylase from the archaebacterium Natronococcus sp. Strain Ah-36. J Bacteriol 174:3439–3444
Kumar CG, Takagi H (1999) Microbial alkaline proteases: from bioindustrial viewpoint. Biotechnol Adv 17:561–594
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lama L, Romano I, Calandrelli V, Nicolaus B, Gambacorta A (2005) Purification and characterization of a protease produced by an aerobic haloalkaliphilic species belonging to the Salinivibrio genus. Res Microbiol 156:478–84
Lippert K, Galinski E (1992) Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing adn drying. Appl Microbial Biotechnol 37:61–65
Madern D, Camacho M, Rodriguez-Arnedo A, Bonete MJ, Zaccai G (2004) Salt-dependent studies of NADP-dependent isocitrate dehydrogense from the halophilic archaeon Haloferax volcanii. Extremophiles 8:377–384
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Miyaji T, Otta Y, Nakagawa T, Watanabe T, Niimura Y, Tomizuka N (2006) Purification and molecular characterization of subtilisin-like alkaline protease BPP-A from Bacillus pumilus strain MS-1. Lett Appl Microbiol 42:242–247
Neklyudov AD, Ivankin AN, Berdutina AV (2000) Properties and uses of protein hydrolysates (review). Appl Biochem Microbiol 36:452–459
Niehaus F, Bertoldo C, Kahler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Nowlan B, Dodia MS, Singh SP, Patel BKC (2006) Bacillus okhensis nov. sp., a halotolerant alkaliphile from an Indian salt pan. Int J Syst Evol Microbiol 56:1073–1077
Patel RK, Dodia MS, Joshi RH, Singh SP (2006) Production of extracellular halo-alkaline protease from a newly isolated Haloalkaliphilic Bacillus sp. isolated from seawater in Western India. World J Microb Biot 24:375–382
Patel RK, Dodia MS, Singh SP (2005) Extracellular alkaline protease from a newly isolated haloalkaliphilic Bacillus sp.: production and optimization. Process Biochem 40:3569–3575
Polosina YY, Zamyatkin DF, Kostyukova AS, Filimonov V, Fedorov OV (2002) Stability of Natrialba magadii NDP kinase: comparisons with other halophilic proteins. Extremophiles 6:135–142
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Sanchez-Porro C., Mellado E., Bertoldo C., Antranikian G., Ventosa A (2003) Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. strain CP76. Extremophiles 7:221–228
Sato M., Yoshikawa K, Minagawa M (1990) The effect of builders on the activity of protease enzymes. J Am Oil Chem Soc 67:711–716
Smacchi E, Fox PF, Gobbetti M (1999) Purification and characterization of two extracellular proteinases from Arthrobacter nicotianae 9458. FEMS Microbiol Lett 170:327–333
Smith CA, Toogood HS, Baker HM, Daniel RM, Baker EN (1999) Calcium-mediated thermostability in Subtilisin superfamily: the crystal structure of Bacillus AK.1 protease at 1.8 Å resolution. J Mol Biol 294:1027–1040
Sorokin DY, Kuenen JG (2005) Chemolithotrophic haloalkaliphiles from soda lakes. FEMS Microbiol Ecol 52:287–95
Sorokin DY, Kuenen JG (2005) Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol Rev 29:685–702
Steele DB, Fiske MJ, Steele BP, Kelley VC (1992) Production of a low molecular weight, alkaline active, thermostable protease by a novel spiral-shaped bacterium, Kurthia spiroforme sp. nov. Enz Microbiol Technol 14:358–360
Stoner MR, Dale DA, Gualfetti PJ, Becker T, Manning MC, Carpenter JF, Randolph TW (2004) Protease autolysis in heavy-duty liquid detergent formulations: effects of thermodynamic stabilizers and protease inhibitors. Enz Microbiol Technol 34:114–125
Studdert CA, Seitz MKH, Gilv MIP, Sanchez JJ, De Castro RE (2001) Purification and biochemical characterization of the haloalkaliphilic archaeon Natronococcus occultus extracellular serine protease. J Basic Microbiol 41:375–383
Studdert CA, De Castro RE, Herrera SK, Sanchez JJ (1997) Detection and preliminary characterization of extracellular proteolytic activities of the haloalkaliphilic archaeon Natronococcus occultus. Arc Microbiol 168:532–535
Tikhonova TV, Slutsky A, Antipov AN, Boyko KM, Polyakov KM, Sorokin DY, Zvyagilskaya RA, Popov VO (2006) Molecular and catalytic properties of a novel cytochrome c nitrite reductase from nitrate-reducing haloalkaliphilic sulfur-oxidizing bacterium Thioalkalivibrio nitratireducens. Biochem Biophys Acta 1764(4):715–723
Tindall BJ, Ross HNM, Grant WD (1984) Natronobacterium gen. nov. and Natronococcus gen. nov., to new genera of haloalkalophilic archaebacteria. Syst Appl Microbiol 5:41–57
Ventosa A, Nieto JJ (1995) Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 11:85–94
Yu TX (1991) Protease of haloalkaliphiles. In: Horikoshi K, Grant WD (eds) Super bugs. Springer, New York, pp 76–83
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dodia, M.S., Rawal, C.M., Bhimani, H.G. et al. Purification and stability characteristics of an alkaline serine protease from a newly isolated Haloalkaliphilic bacterium sp. AH-6. J Ind Microbiol Biotechnol 35, 121–131 (2008). https://doi.org/10.1007/s10295-007-0273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0273-x