Abstract
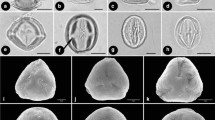
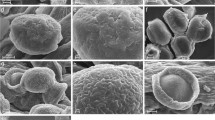
The pollen morphology of 11 genera and 11 species of the Hydrocharitaceae and one species of the Najadaceae was studied using scanning and transmission electron microscopies, and the exine structures and sculptures are discussed in relation to pollination mechanisms and the molecular phylogeny. The pollen grains of the Hydrocharitaceae are spherical, inaperturate, and form monads or tetrads, while those of the Najadaceae are elliptical, inaperturate, and form monads. The entomophilous genera Egeria, Blyxa, Ottelia, Stratiotes, and Hydrocharis share pollen grains that have projections like spines or bacula. The anemophilous genus Limnobium has reticulate pollen grains. The hypohydrophilous genera Thalassia and Najas are characterized by pollen grains with reduced exine structures. The pollen-epihydrophilous genera Elodea and Hydrilla have tightly arranged small spinous pollen grains, and the male flower-epihydrophilous genera Enhalus and Vallisneria have reduced reticulate or gemmate exines. Character state reconstruction of the exine structures and sculptures using a molecular phylogenetic tree suggests that variation in the exine is generally correlated with the pollination mechanism; the selective pressures acting on the pollination mechanisms have reduced the exine structure in hypohydrophilous plants and resulted in various exine sculptures that are adapted to the different pollination mechanisms in entomophilous, anemophilous, and pollen-epihydrophilous plants.







Similar content being viewed by others
References
Angiosperm Phylogeny Group (2003) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
Cook CDK (1982) Pollination mechanisms in the Hydrocharitaceae. In: Symoens JJ, Hooper SS, Compere P (eds) Studies on aquatic vascular plants. Royal Botanical Society of Belgium, Brussels, pp 1–15
Cook CDK (1985) A revision of the genus Apalanthe (Hydrocharitaceae). Aquat Bot 21:157–164
Cook CDK (1988) Wind pollination in aquatic angiosperms. Ann Mo Bot Gard 75:768–777
Cook CDK (1996) Aquatic plant book. SPB, Amsterdam
Cook CDK, Luond R (1982a) A revision of the genus Nechamandra (Hydrocharitaceae). Aquat Bot 13:505–513
Cook CDK, Luond R (1982b) A revision of the genus Hydrocharis (Hydrocharitaceae). Aquat Bot 14:177–204
Cook CDK, Luond R (1983) A revision of the genus Blyxa (Hydrocharitaceae). Aquat Bot 15:1–52
Cook CDK, Urmi-Koenig K (1983) A revision of the genus Limnobium including Hydromystria (Hydrocharitaceae). Aquat Bot 17:1–27
Cook CDK, Urmi-Koenig K (1984) A revision of the genus Egeria (Hydrocharitaceae). Aquat Bot 19:73–96
Cook CDK, Urmi-Koenig K (1985) A revision of the genus Elodea (Hydrocharitaceae). Aquat Bot 21:111–156
Cook CDK, Symoens J, Urmi-Koenig K (1984) A revision of the genus Ottelia (Hydrocharitaceae). I. Generic considerations. Aquat Bot 18:263–274
Crane PR, Fris EM, Pedersen KR (1995) The origin and early diversification of angiosperms. Nature 374:27–33
Dahlgren RMT, Clifford HT, Yeo PF (1985) The families of the monocotyledons. Structure, evolution, and taxonomy. Springer, Berlin Heidelberg New York
Erdtman G (1960) The acetolysis method. A revised description. Sven Bot Tidskr 54:561–564
Furness CA, Rudall PJ (1999) Inaperturate pollen in Monocotyledons. Int J Plant Sci 160:395–414
Grayum MH (1992) Comparative external pollen ultrastructure of the Araceae and putatively related taxa. Missouri Botanical Garden, St. Louis, Missouri
Harley MM, Kurmann MH, Ferguson IK (1991) Systematic implications of comparative morphology in selected Tertiary and extant pollen from the Palmae and the Sapotaceae. Pollen Spores 44:225–238
Heslop-Harrison J (1976) The adaptive significance of the exine. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine. Linnean Society Symposium Series 1. The Linnean Society of London, London, pp 27–37
Hesse M (2000) Pollen wall stratification and pollination. Plant Syst Evol 222:1–17
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A–138A
Kaul RB (1970) Evolution and adaptation of inflorescences in the Hydrocharitaceae. Am J Bot 57:708–715
Kress WJ (1986) Exineless pollen structure and pollination systems of tropical Heliconia (Heliconiaceae). In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. The Linnean Society of London, London, pp 329–345
Kress WJ, Stone DE (1978) Ultrastructure of exine-less pollen; Heliconia (Heliconiaceae). Am J Bot 65:1064–1076
Les DH, Cleland MA, Waycott M (1997) Phylogenetic studies in Alismatidae. II. Evolution of marine angiosperms (seagrasses) and hydrophily. Syst Bot 22:1–21
Madisson WP, Maddison DR (1992) MacClade: analysis of phylogeny and character evolution. Version 3.0. Sinauer, Sunderland, Mass.
Mayo SJ, Bogner J, Boyce PC (1997) The genera of Araceae. Royal Botanic Gardens, Kew
Nilsson S (1990) Taxonomic and evolutionary significance of pollen morphology in the Apocynaceae. Plant Syst Evol 5[Suppl]:91–102
Pettitt JM (1980) Reproduction in seagrass: nature of the pollen and receptive surface of the stigma in the Hydrocharitaceae. Ann Bot 45:257–271
Pettitt JM (1981) Reproduction in seagrasses: pollen development in Thalassia hemprighii, Halophila stipulacea and Thalassodendron ciliatum. Ann Bot 48:609–622
Pettitt JM, Jermy AC (1975) Pollen in hydrophilous angiosperms. Micron 5:377–405
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Harper Collins, London
Scribailo RW, Posluszny U (1984) The reproductive biology of Hydrocharis morsus-ranae. I. Floral biology. Can J Bot 62:2779–2787
Skvarla JJ, Rowley JR (1970) The pollen wall of Canna and its similarity to the germinal apertures of other pollen. Am J Bot 57: 519–529
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Takahashi M (1982) Pollen morphology in North American species of Trillium. Am J Bot 69:1185–1195
Takahashi M (1983) Pollen morphology in Asiatic species of Trillium. Bot Mag (Tokyo) 96:377–384
Takahashi M (1987) Development of omniaperturate pollen in Trillium kamtchaticum (Liliaceae). Am J Bot 74:1842–1852
Takahashi M (1994) Pollen development in a submerged plant, Ottelia aismoides (L. ) Pers. (Hydrocharitaceae). J Plant Res 107:161–164
Takahashi M (1995) Development of structure-less pollen wall in Ceratophyllum demersum L. (Ceratophyllaceae). J Plant Res 108:205–208
Tanaka N (2000) Pollination of the genus Hydrilla (Hydrocharitaceae) by waterborne pollen grains. Ann Tsukuba Bot Gard 19:7–12
Tanaka N (2003) Pollination of the genus Hydrilla (Hydrocharitaceae) by waterborne pollen grains. II. Air bubbles cause the male flower to surface. Ann Tsukuba Bot Gard 22:11–13
Tanaka N, Setoguchi H, Murata J (1997) Phylogeny of the family Hydrocharitaceae inferred from rbcL and matK gene sequence data. J Plant Res 110:329–337
Verhoeven JTA (1979) The ecology of Ruppia dominated communities in western Europe. I. Distribution of Ruppia representatives in relation to their autecology. Aquat Bot 6:197–268
Walker JW (1976) Evolutionary significance of the exine in the pollen of primitive angiosperms. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine. Linnean Symposium Society Series 1. Academic, New York, pp 251–308
Whitehead DR (1968) Wind pollination in the angiosperms: evolutionary and environmental considerations. Evolution 23:28–35
Wodehouse RP (1935) Pollen grains. McGraw-Hill, New York
Zavada MS (1983) Comparative morphology of monocot pollen and evolutionary trends of apertures and wall structures. Bot Rev 49:331–379
Acknowledgements
The authors thank Michio Wakabayashi and Hiroaki Setoguchi for critical reading of the manuscript and comments; Isao Uemura for technical guidance; Madjit Hakki, Yasuro Kadono, Keiko Aioi, Yuji Omori, Toshie Kikuchi, Yasuo Orime, Takashi Enomoto and Yuki Mikanagi for collecting plant materials; and Eitetsu Sugiyama and Takako Yasugi for cultivating plants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, N., Uehara, K. & Murata, J. Correlation between pollen morphology and pollination mechanisms in the Hydrocharitaceae. J Plant Res 117, 265–276 (2004). https://doi.org/10.1007/s10265-004-0155-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-004-0155-5