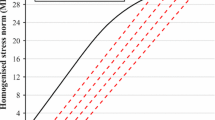
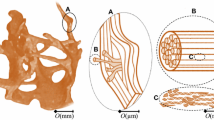
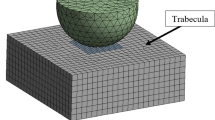
Abstract
Trabecular bone tissue failure can be considered as consisting of two stages: damage and fracture; however, most failure analyses of 3D high-resolution trabecular bone samples are confined to damage mechanisms only, that is, without fracture. This study aims to develop a computational model of trabecular bone consisting of an explicit representation of complete failure, incorporating damage criteria, fracture criteria, cohesive forces, asymmetry and large deformation capabilities. Following parameter studies on a test specimen, and experimental testing of bone sample to complete failure, the asymmetric critical tissue damage and fracture strains of ovine vertebral trabecular bone were calibrated and validated to be compression damage −1.16 %, tension damage 0.69 %, compression fracture −2.91 % and tension fracture 1.98 %. Ultimate strength and post–ultimate strength softening were captured by the computational model, and the failure of individual struts in bending and shear was also predicted. This modelling approach incorporated a cohesive parameter that provided a facility to calibrate ductile–brittle behaviour of bone tissue in this non-linear geometric and non-linear constitutive property analyses tool. Finally, the full accumulation of tissue damage and tissue fracture has been monitored from range of small magnitude (normal daily loading) through to specimen yielding, ultimate strength and post–ultimate strength softening.
Similar content being viewed by others
References
Barenblatt GI (1962) The mathematical theory of equilibrium cracks in brittle fracture. Adv Appl Mech 7: 55–129
Bayraktar HH, Keaveny TM (2004) Mechanisms of uniformity of yield strains for trabecular bone. J Biomech 37(11): 1671–1678
Bevill G, Keaveny TM (2009) Trabecular bone strength predictions using finite element analysis of micro-scale images at limited spatial resolution. Bone 44(4): 579–584
Burr DB, Martin RB, Schaffler MB, Radin EL (1985) Bone remodeling in response to in vivo fatigue microdamage. J Biomech 18(3): 189–200
Charlebois M, Jirásek M, Zysset P (2010) A nonlocal constitutive model for trabecular bone softening in compression. Biomech Model Mechanobiol 9(5): 597–611. doi:10.1007/s10237-010-0200-3
de Borst R (2002) Fracture in quasi-brittle materials: a review of continuum damage-based approaches. Eng Fract Mech 69(2): 95–112
De Santis R, Anderson P, Tanner KE, Ambrioso L, Nicolais L, Bonfield W, Davis GR (2000) Bone fracture analysis on the short rod chevron-notch specimens using the X-ray computer micro-tomography. J Mater Sci Mater Med 11(10): 629–636
Dendorfer S, Maier HJ, Hammer J (2009) Fatigue damage in cancellous bone: an experimental approach from continuum to micro scale. J Mech Behav Biomed Mater 2(1): 113–119
Dugdale DS (1960) Yielding of steel sheets containing slits. J Mech Phys Solids 8(2): 100–104
Elices M, Guinea GV, Gomez J, Planas J (2002) The cohesive zone model: advantages, limitations and challenges. Eng Fract Mech 69(2): 137–163
Fazzalari NL, Forwood MR, Manthey BA, Smith K, Kolesik P (1998) Three-dimensional confocal images of microdamage in cancellous bone. Bone 23(4): 373–378
Fyhrie DP, Schaffler MB (1994) Failure mechanisms in human vertebral cancellous bone. Bone 15(1): 105–109
Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA (1994) The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech 27(4): 375–377
Guldberg RE, Hollister SJ (1994) Finite element solution errors associated with digital image-based mesh generation. Adv Bioeng Am Soc Mech Eng Bioeng Div (publication) BED 28: 147–148
Guldberg RE, Hollister SJ, Charras GT (1998) The accuracy of digital image-based finite element models. J Biomech Eng 120(2): 289–295
Gupta HS, Zioupos P (2008) Fracture of bone tissue: The [‘]hows’ and the [‘]whys’. Med Eng Phys 30(10): 1209–1226
Harrigan TP, Jasty M, Mann RW, Harris WH (1988) Limitations of the continuum assumption in cancellous bone. J Biomech 21(4): 269–275
Harrison N, McHugh P (2010) Comparison of trabecular bone behavior in core and whole bone samples using high-resolution modeling of a vertebral body. Biomech Model Mechanobiol 9(4): 469–480. doi:10.1007/s10237-009-0188-8
Harrison N, O’Mahoney D, McDonnell P, McHugh P (2006) Damage and failure of trabecular bone with non-linear geometry and inhomogeneous material properties. J Biomech (abstracts of the 5th world Congress of Biomechanics) 39(suppl 1): S417
Harrison NM (2007) Small and large deformation modelling of trabecular bone based on the voxel finite element method. Ph.D. thesis, National University of Ireland Galway, Galway
Harrison NM, McDonnell PF, O’Mahoney DC, Kennedy OD, O’Brien FJ, McHugh PE (2008) Heterogeneous linear elastic trabecular bone modelling using micro-CT attenuation data and experimentally measured heterogeneous tissue properties. J Biomech 41(11): 2589–2596
Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK (2004) High-resolution AFM imaging of intact and fractured trabecular bone. Bone 35(1): 4–10. doi:10.1016/j.bone.2004.02.024
Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM (2005) Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37(6): 825–832
Hillerborg A, Modéer M, Petersson PE (1976) Analysis of crack formation and crack growth in concrete by means of fracture mechanics and finite elements. Cem Concr Res 6: 773–782
Ichim I, Li Q, Li W, Swain MV, Kieser J (2007) Modelling of fracture behaviour in biomaterials. Biomaterials 28(7): 1317–1326
Johnell O, Kanis J (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12): 1726–1733. doi:10.1007/s00198-006-0172-4
Keaveny TM, Pinilla TP, Crawford RP, Kopperdahl DL, Lou A (1997) Systematic and random errors in compression testing of trabecular bone. J Orthop Res 15(1): 101–110
Keller TS (1994) Predicting the compressive mechanical behavior of bone. J Biomech 27(9): 1159–1168
Kim D-G, Christopherson GT, Dong XN, Fyhrie DP, Yeni YN (2004) The effect of microcomputed tomography scanning and reconstruction voxel size on the accuracy of stereological measurements in human cancellous bone. Bone 35(6): 1375–1382
Kiusalaas J (2010) Interpolation and curve fitting. In: Numerical methods in engineering with python. Cambridge University Press, New York, NY, USA
Kopperdahl DL, Keaveny TM (1998) Yield strain behavior of trabecular bone. J Biomech 31(7): 601–608
Kosmopoulos V, Keller TS (2008) Predicting trabecular bone microdamage initiation and accumulation using a non-linear perfect damage model. Med Eng Phys 30(6): 725–732
Kosmopoulos V, Schizas C, Keller TS (2008) Modeling the onset and propagation of trabecular bone microdamage during low-cycle fatigue. J Biomech 41(3): 515–522
Ladd AJC, Kinney JH (1998) Numerical errors and uncertainties in finite-element modeling of trabecular bone. J Biomech 31(10): 941–945
Lee TC, Staines A, Taylor D (2002) Bone adaptation to load: microdamage as a stimulus for bone remodelling. J Anat 201(6): 437–446
Leng H, Wang X, Ross RD, Niebur GL, Roeder RK (2008) Micro-computed tomography of fatigue microdamage in cortical bone using a barium sulfate contrast agent. J Mech Behav Biomed Mater 1(1): 68–75
Lotz JC, Gerhart TN, Hayes WC (1990) Mechanical properties of trabecular bone from the proximal femur: a quantitative CT study. J Comput Assist Tomogr 14(1): 107–114
MacNeil JA, Boyd SK (2008) Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone 42(6): 1203–1213
Mc Donnell P, Harrison N, Liebschner MAK, Mc Hugh PE (2009) Simulation of vertebral trabecular bone loss using voxel finite element analysis. J Biomech 42(16): 2789–2796
Mc Donnell P, Harrison N, Lohfeld S, Kennedy O, Zhang Y, Mc Hugh PE (2010a) Investigation of the mechanical interaction of the trabecular core with an external shell using rapid prototype and finite element models. J Mech Behav Biomed Mater 3(1): 63–76
McDonnell P, Harrison N, McHugh PE (2010b) Investigation of the failure behaviour of vertebral trabecular architectures under uni-axial compression and wedge action loading conditions. Med Eng Phys 32(6): 569–576
McNamara LM, Ederveen AGH, Lyons CG, Price C, Schaffler MB, Weinans H, Prendergast PJ (2006) Strength of cancellous bone trabecular tissue from normal, ovariectomized and drug-treated rats over the course of ageing. Bone 39(2): 392–400
McNamara LM, Prendergast PJ (2007) Bone remodelling algorithms incorporating both strain and microdamage stimuli. J Biomech 40(6): 1381–1391
Mercer C, He MY, Wang R, Evans AG (2006) Mechanisms governing the inelastic deformation of cortical bone and application to trabecular bone. Acta Biomaterialia 2(1): 59–68
Morgan EF, Keaveny TM (2001) Dependence of yield strain of human trabecular bone on anatomic site. J Biomech 34: 569–577
Nalla RK, Kinney JH, Ritchie RO (2003) Mechanistic fracture criteria for the failure of human cortical bone. Nat Mater 2(3): 164–168
Nalla RK, Stolken JS, Kinney JH, Ritchie RO (2005) Fracture in human cortical bone: local fracture criteria and toughening mechanisms. J Biomech 38(7): 1517–1525
Niebur GL, Feldstein MJ, Keaveny TM (2002) Biaxial failure behavior of bovine tibial trabecular bone. J Biomech Eng 124(6): 699–705
Niebur GL, Feldstein MJ, Yuen JC, Chen TJ, Keaveny TM (2000) High-resolution finite element models with tissue strength asymmetry accurately predict failure of trabecular bone. J Biomech 33(12): 1575–1583
Niebur GL, Yuen JC, Burghardt AJ, Keaveny TM (2001) Sensitivity of damage predictions to tissue level yield properties and apparent loading conditions. J Biomech 34(5): 699–706
O’Brien FJ, Taylor D, Lee TC (2002) An improved labelling technique for monitoring microcrack growth in compact bone. J Biomech 35((4): 523–526
Osteoporosis-International: (1997) Who are candidates for prevention and treatment for osteoporosis. Osteoporos Int 7(1): 1–6. doi:10.1007/bf01623453
Pistoia W, Rietbergen Bv, Laib A, Ruegsegger P (2001) High-resolution three-dimensional-pQCT images can be an adequate basis for in-vivo mu FE analysis of bone. J Biomech Eng 123(2): 176–183
Reilly GC, Currey JD (2000) The effects of damage and microcracking on the impact strength of bone. J Biomech 33(3): 337–343
Rohlmann A, Zilch H, Bergmann G, Kolbel R (1980) Material properties of femoral cancellous bone in axial loading. Part I: time independent properties. Arch Orthop Trauma Surg 97(2): 95–102
Scheider I, Brocks W (2003) Simulation of cup-cone fracture using the cohesive model. Eng Fract Mech 70(14): 1943–1961
Schileo E, Taddei F, Cristofolini L, Viceconti M (2008) Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J Biomech 41(2): 356–367
Shi X, Sherry Liu X, Wang X, Edward Guo X, Niebur GL (2010) Type and orientation of yielded trabeculae during overloading of trabecular bone along orthogonal directions. J Biomech 43(13): 2460–2466
Shi X, Wang X, Niebur G (2009) Effects of loading orientation on the morphology of the predicted yielded regions in trabecular bone. Ann Biomed Eng 37((2): 354–362
Stops AJF, Harrison NM, Haugh MG, O’Brien FJ, McHugh PE (2010) Local and regional mechanical characterisation of a collagen-glycosaminoglycan scaffold using high-resolution finite element analysis. J Mech Behav Biomed Mater 3(4): 292–302
Tang SY, Vashishth D (2007) A non-invasive in vitro technique for the three-dimensional quantification of microdamage in trabecular bone. Bone 40((5): 1259–1264
Taylor D (2003) Fracture mechanics: how does bone break?. Nat Mater 2(3): 133–134
Thurner PJ, Erickson B, Jungmann R, Schriock Z, Weaver JC, Fantner GE, Schitter G, Morse DE, Hansma PK (2007) High-speed photography of compressed human trabecular bone correlates whitening to microscopic damage. Eng Fract Mech (first international conference on the mechanics of biomaterials and tissues) 74(12): 1928–1941
Thurner PJ, Wyss P, Voide R, Stauber M, Stampanoni M, Sennhauser U, Muller R (2006) Time-lapsed investigation of three-dimensional failure and damage accumulation in trabecular bone using synchrotron light. Bone 39(2): 289–299
Tomar V (2008) Modeling of dynamic fracture and damage in two-dimensional trabecular bone microstructures using the cohesive finite element method. J Biomech Eng 130(2): 021021
Tomar V (2009) Insights into the effects of tensile and compressive loadings on microstructure dependent fracture of trabecular bone. Eng Fract Mech 76(7): 884–897
Ulrich D, van Rietbergen B, Weinans H, Ruegsegger P (1998) Finite element analysis of trabecular bone structure: a comparison of image-based meshing techniques. J Biomech 31(12): 1187–1192
Ural A, Vashishth D (2006) Cohesive finite element modeling of age-related toughness loss in human cortical bone. J Biomech 39(16): 2974–2982
Vashishth D, Behiri JC, Bonfield W (1997) Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech 30(8): 763–769
Verhulp E, van Rietbergen B, Muller R, Hulskes R (2008) Indirect determination of trabecular bone effective tissue properties using micro-finite element simulations. J Biomech 41: 1479–1485. doi:10.1016/j.jbiomech.2008.02.032
Wachtel EF, Keaveny TM (1997) Dependence of trabecular damage on mechanical strain. J Orthop Res 15(5): 781–787
Wang X, Niebur GL (2006) Microdamage propagation in trabecular bone due to changes in loading mode. J Biomech 39(5): 781–790
Wang X, Zauel RR, Rao DS, Fyhrie DP (2008) Cancellous bone lamellae strongly affect microcrack propagation and apparent mechanical properties: Separation of patients with osteoporotic fracture from normal controls using a 2D nonlinear finite element method (biomechanical stereology). Bone 42(6): 1184–1192
Yang QD, Cox BN, Nalla RK, Ritchie RO (2006) Fracture length scales in human cortical bone: the necessity of nonlinear fracture models. Biomaterials 27(9): 2095–2113
Yeh OC, Keaveny TM (2001) Relative roles of microdamage and microfracture in the mechanical behavior of trabecular bone. J Orthop Res 19(6): 1001–1007
Yeni YN, Fyhrie DP (2003) A rate-dependent microcrack-bridging model that can explain the strain rate dependency of cortical bone apparent yield strength. J Biomech 36(9): 1343–1353
Zioupos P, Currey JD (1994) The extent of microcracking and the morphology of microcracks in damaged bone. J Mater Sci 29(4): 978–986
Zioupos P, Currey JD (1998) Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 22(1): 57–66
Zioupos P, Currey JD, Mirza MS, Barton DC (1995) Experimentally determined microcracking around a circular hole in a flat plate of bone: comparison with predicted stresses. Philos Trans R Soc B Biol Sci 347(1322): 383–396
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrison, N.M., McDonnell, P., Mullins, L. et al. Failure modelling of trabecular bone using a non-linear combined damage and fracture voxel finite element approach. Biomech Model Mechanobiol 12, 225–241 (2013). https://doi.org/10.1007/s10237-012-0394-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-012-0394-7