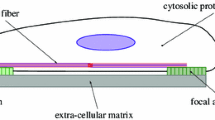
Abstract
We have developed a three-dimensional random network model of the intracellular actin cytoskeleton and have used it to study the role of the cytoskeleton in mechanotransduction and nucleus deformation. We use the model to predict the deformation of the nucleus when mechanical stresses applied on the plasma membrane are propagated through the random cytoskeletal network to the nucleus membrane. We found that our results agree with previous experiments utilizing micropipette pulling. Therefore, we propose that stress propagation through the random cytoskeletal network can be a mechanism to effect nucleus deformation, without invoking any biochemical signaling activity. Using our model, we also predict how nucleus strain and its relative displacement within the cytosol vary with varying concentrations of actin filaments and actin-binding proteins. We find that nucleus strain varies in a sigmoidal manner with actin filament concentration, while there exists an optimal concentration of actin-binding proteins that maximize nucleus displacement. We provide a theoretical analysis for these nonlinearities in terms of the connectivity of the random cytoskeletal network. Finally, we discuss laser ablation experiments that can be performed to validate these results in order to advance our understanding of the role of the cytoskeleton in mechanotransduction.
Similar content being viewed by others
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Caille N, Thoumine O, Tardy Y, Meister JJ (2002) Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35(2): 177–187
Chien S (2007) Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292(3): H1209–H1214
Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, Stelzer EHK (2009) Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci 122(Pt 10): 1665–1669
Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172(1): 41–53
Dalby M (2005) Topographically induced direct cell mechanotransduction. Med Eng Phys 27(9): 730–742
Dalby MJ, Riehle MO, Sutherland DS, Agheli H, Curtis ASG (2004) Use of nanotopography to study mechanotransduction in fibroblasts-methods and perspectives. Eur J Cell Biol 83(4): 159–169
Day AR, Tremblay RR, Tremblay AMS (1986) Rigid backbone: a new geometry for percolation. Phys Rev Lett 56(23): 2501–2504
Doddi SK, Bagchi P (2009) Three-dimensional computational modeling of multiple deformable cells flowing in microvessels. Phys Rev E Stat Nonlinear Soft Matter Phys 79(4 Pt 2): 046,318
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4): 677–679
Evans EA, Skalak R (1979a) Mechanics and thermodynamics of biomembranes: part 1. CRC Crit Rev Bioeng 3(3): 181–330
Evans EA, Skalak R (1979b) Mechanics and thermodynamics of biomembranes: part 2. CRC Crit Rev Bioeng 3(4): 331–418
Feng S, Sen PN (1984) Percolation on elastic networks: new exponent and threshold. Phys Rev Lett 52(3): 216–219
Forgacs G (1995) On the possible role of cytoskeletal filamentous networks in intracellular signaling: an approach based on percolation. J Cell Sci 108(6): 2131–2144
Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA (2004) Elastic behavior of cross-linked and bundled actin networks. Science 304(5675): 1301–1305
Gardel ML, Nakamura F, Hartwig J, Crocker JC, Stossel TP, Weitz DA (2006a) Stress-dependent elasticity of composite actin networks as a model for cell behavior. Phys Rev Lett 96(8): 088,102
Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA (2006b) Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA 103(6): 1762–1767
Giannone G, Sheetz MP (2006) Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol 16(4): 213–223
Gudi S, Nolan JP, Frangos JA (1998) Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95(5): 2515–2519
Guo W, Frey MT, Burnham NA, li Wang Y (2006) Substrate rigidity regulates the formation and maintenance of tissues. Biophys J 90(6): 2213–2220
Head DA, Levine AJ, MacKintosh FC (2003) Distinct regimes of elastic response and deformation modes of cross-linked cytoskeletal and semiflexible polymer networks. Phys Rev E Stat Nonlinear Soft Matt Phys 68(6 Pt 1): 061,907
Huisman EM, van Dillen T, Onck PR, Vander Giessen E (2007) Three-dimensional cross-linked F-actin networks: relation between network architecture and mechanical behavior. Phys Rev Lett 99(20): 208,103
Jean RP, Chen CS, Spector AA (2005) Finite-element analysis of the adhesion-cytoskeleton-nucleus mechanotransduction pathway during endothelial cell rounding: axisymmetric model. J Biomech Eng 127(4): 594–600
Kim T, Hwang W, Lee H, Kamm RD (2009) Computational analysis of viscoelastic properties of crosslinked actin networks. PLoS Comput Biol 5(7): e1000,439
Liu WF, Nelson CM, Tan JL, Chen CS (2007) Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res 101(5): e44–e52
Lo CM, Wang HB, Dembo M, Wang YL (2000) Cell movement is guided by the rigidity of the substrate. Biophys J 79(1): 144–152
Maniotis AJ, Chen CS, Ingber DE (1997) Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA 94(3): 849–854
Marcelli G, Parker KH, Winlove CP (2005) Thermal fluctuations of red blood cell membrane via a constant-area particle-dynamics model. Biophys J 89(4): 2473–2480
Martinac B (2004) Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci 117(Pt 12): 2449–2450
Mastro AM, Babich MA, Taylor WD, Keith AD (1984) Diffusion of a small molecule in the cytoplasm of mammalian cells. Proc Natl Acad Sci USA 81(11): 3414–3418
Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T (2008) Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27(23): 3092–3093
Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N (2008) Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA 105(18): 6626–6631
Nalepa G, Harper JW (2004) Visualization of a highly organized intranuclear network of filaments in living mammalian cells. Cell Motil Cytoskelet 59(2): 94–108
Onck PR, Koeman T, van Dillen T, Van der Giessen E (2005) Alternative explanation of stiffening in cross-linked semiflexible networks. Phys Rev Lett 95(17): 178,102
Palmer JS, Boyce MC (2008) Constitutive modeling of the stress–strain behavior of F-actin filament networks. Acta Biomater 4(3): 597–612
Sato M, Levesque MJ, Nerem RM (1987) An application of the micropipette technique to the measurement of the mechanical properties of cultured bovine aortic endothelial cells. J Biomech Eng 109(1): 27–34
Shafrir Y, Forgacs G (2002) Mechanotransduction through the cytoskeleton. Am J Physiol Cell Physiol 282(3): C479–C486
Shen N, Datta D, Schaffer CB, LeDuc P, Ingber DE, Mazur E (2005) Ablation of cytoskeletal filaments and mitochondria in live cells using a femtosecond laser nanoscissor. Mech Chem Biosyst 2(1): 17–25
Shin JH, Gardel ML, Mahadevan L, Matsudaira P, Weitz DA (2004) Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc Natl Acad Sci USA 101(26): 9636–9641
Stewart CL, Roux KJ, Burke B (2007) Blurring the boundary: the nuclear envelope extends its reach. Science 318(5855): 1408–1412
Tetsunaga T, Furumatsu T, Abe N, Nishida K, Naruse K, Ozaki T (2009) Mechanical stretch stimulates integrin alphaVbeta3-mediated collagen expression in human anterior cruciate ligament cells. J Biomech 42(13): 2097–2103
Thery M, Pepin A, Dressaire E, Chen Y, Bornens M (2006) Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil cytoskelet 63(6): 341–345
Thomas CH, Collier JH, Sfeir CS, Healy KE (2002) Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci USA 99(4): 1972–1977
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437(7057): 426–431
Wang N, Tytell JD, Ingber DE (2009) Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10(1): 75–82
Wiesner TF, Berk BC, Nerem RM (1997) A mathematical model of the cytosolic-free calcium response in endothelial cells to fluid shear stress. Proc Natl Acad Sci USA 94(8): 3726–3731
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, Y., Yip, A.K., Teo, SK. et al. A three-dimensional random network model of the cytoskeleton and its role in mechanotransduction and nucleus deformation. Biomech Model Mechanobiol 11, 49–59 (2012). https://doi.org/10.1007/s10237-011-0292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0292-4