Abstract
The best treatment strategy for severe acute respiratory syndrome (SARS) is still unknown. Ribavirin and corticosteroids were used extensively during the SARS outbreak. Ribavirin has been criticized for its lack of efficacy. Corticosteroids are effective in lowering the fever and reversing changes in the chest radiograph but have the caveat of encouraging viral replication. The effectiveness of corticosteroids has only been suggested by uncontrolled observations, and the role of these agents in therapy remains to be established by randomized controlled studies. Both ribavirin and corticosteroids have very significant side effects. The lopinavir/ritonavir combination has been shown to reduce the intubation rate and the incidence of adverse clinical outcomes when used with ribavirin. When patients deteriorate clinically despite treatment with ribavirin and corticosteroids, rescue treatment with convalescent plasma and immunoglobulin may be beneficial. Noninvasive positive pressure ventilation is a sound treatment for SARS patients with respiratory failure if administered with due precaution in the correct environment. Interferons and other novel agents may hold promise as useful anti-SARS therapies in the future. The experience with traditional Chinese medicine is encouraging, and its use as an adjuvant should be further investigated.
Similar content being viewed by others
Introduction
A global outbreak of severe acute respiratory syndrome (SARS) started in March 2003 and was caused by the SARS-associated coronavirus (SARS-CoV) [1–3]. The cumulative number of cases in 29 countries and regions up to 31 July 2003 was 8,096. The total number of deaths was 774, with a case–fatality ratio of 9.6% [4]. Owing to the lack of knowledge about this new and serious disease, different treatment modalities have been used empirically with much uncertainty. The overwhelming situation of large numbers of ill patients deteriorating rapidly prevented anxious physicians from performing randomized controlled treatment trials [5], which were regarded as dangerous and unethical in the face of a life-threatening condition.
Many treatment options, including antiviral agents, immunosuppressive agents, convalescent plasma, immunoglobulin, noninvasive positive pressure ventilation (NIPPV), and traditional Chinese medicine (TCM), have been introduced on the basis of different rationales. First, SARS has an initial viral replicative phase, which peaks at around day 10. Response to this viral load takes the form of inflammatory cell infiltration of tissues and overproduction of cytokines, resulting in immunopathological damage. Thus, there is a therapeutic window that can be exploited to prevent disease progression if a potent antiviral agent is available. Ribavirin has been chosen for use due to its wide spectrum of activity, despite being rather weak against SARS-CoV. Protease inhibitors are now under study because of the experimental evidence that they can inhibit the 3C-like (3CL) protease, which is essential for the life cycle of the SARS-CoV [6, 7]. Second, corticosteroids have been utilized to mitigate the tissue-damaging effects of inflammatory cells and cytokines and to treat the acute respiratory distress syndrome (ARDS) [8], bronchiolitis obliterans organizing pneumonia (BOOP) [9], and septic shock [10] that may occur. Third, convalescent plasma has been administered in the belief that the large amount of neutralizing antibodies present can block the action of the SARS-CoV. Fourth, immunoglobulin has been employed as a salvage therapy in patients with inexorable deterioration despite usual treatment. This approach is based on the immunomodulatory effect of immunoglobulin, which includes competitive occupation of macrophage receptors, neutralization of activated complements, cytokines and superantigens, and inhibition of activated T lymphocytes. Fifth, the application of NIPPV in SARS originates from its usefulness in the management of patients with chronic obstructive airways disease complicated by severe community-acquired pneumonia. Sixth, TCM has been used in China because of its known efficacy in the treatment of “fevers” throughout the ages. With the absence of a consensus on the optimal therapeutic strategy, various approaches have been devised elsewhere in the world.
Antiviral agents
Antiviral therapy was adopted at the start of the Hong Kong outbreak because SARS was considered to be a viral infection even before the identification of the SARS-CoV. Detection of the SARS-CoV in the early phase of the disease has been found to be a poor prognostic sign. In one retrospective case analysis, patients with a positive RT-PCR test in nasopharyngeal aspirate samples for SARS-CoV on admission, a mean of 4.3 days after the onset of illness, were more likely to have adverse outcomes in terms of survival, ICU care, and assisted ventilation when compared to patients with negative RT-PCR results [11]. A prospective study revealed that there is an initial viral replicative phase followed by an immunopathological phase and an end organ-damage phase [12]. It is hypothesized that the viral antigen triggers the immune response and that the best approach will be to halt the early viral replication so as to diminish the peak viral load and immunopathological damage. This will result in a lessened requirement for immunosuppressants, which may predispose patients to nosocomial infections, particularly those on mechanical ventilation.
Ribavirin was chosen for use empirically due to its broad antiviral spectrum [13] and the assumption that it could provide coverage for the corticosteroid treatment, which was widely utilized at that time. In the end, over 90% of the patients in Hong Kong received ribavirin. The mechanism of action of this nucleoside analogue has not been well established [14]. Ribavirin has attracted considerable criticism because of its relative lack of in vitro activity against the SARS-CoV [15–17]. Very high concentrations of ribavirin are needed to inhibit the virus transiently, and the levels are difficult to achieve clinically [18]. In a retrospective, uncontrolled cohort analysis, the use of ribavirin did not appear to confer any benefit to SARS patients [19]. Ribavirin is associated with a number of significant adverse effects. The most important one is anemia [12, 20, 21], with one study reporting a drop in hemoglobin of 2 g/dl or more in 49% of patients [20]. Anemia is related to hemolysis in most circumstances [16, 20, 22, 23]. Oxygen desaturation and tissue hypoxia are exacerbated by the reduced oxygen carriage capacity of low hemoglobin. Other important side effects include bradycardia (14% of patients) [20], raised serum transaminases (40% of patients) [20], and teratogenic potential [24]. Ribavirin, given together with corticosteroids, was shown to be incapable of preventing the peaking of viral load on day 10 after the onset of illness [25]. Thus, ribavirin is a controversial drug [26, 27] with low efficacy and significant toxicities. There is an urgent need to find more potent and safer antiviral agents.
In the quest for effective agents against the SARS-CoV, the protease inhibitor lopinavir, when combined with ribavirin, has been found to reach the synergistic inhibitory concentration against the SARS-CoV in laboratory testing [12]. Lopinavir, in combination with ritonavir (LPV/r), is licensed for the treatment of HIV infection. Ritonavir is a weak antiviral agent, but it increases the serum level of lopinavir through inhibition of the CYP3A-mediated metabolism of its partner. Though a satisfactory serum inhibitory concentration of lopinavir may not be achieved with the oral LPV/r combination [28], a sufficient level may be reached at the intestinal mucosa, since 20% of the drug is found excreted unchanged in the stool [29]. This is significant because severe watery diarrhea is a marked feature in 20.4–76.0% of SARS patients [12, 25, 30, 31] and the gastrointestinal mucosa could be an important reservoir for viral replication and fecal-oral transmission [32]. In an open trial of the use of the LPV/r combination together with ribavirin in 41 patients compared with 111 historical controls, it was shown that patients in the LPV/r group had fewer 21-day adverse clinical outcomes (death/acute respiratory distress syndrome) and their disease course was milder with regard to diarrhea, fever recurrence, and worsening of chest radiographs. In addition, the LPV/r group had a progressive decrease in the viral load, an early rise in the lymphocyte count, a reduction in the cumulative dose of pulse methylprednisolone, and fewer episodes of nosocomial infections [21]. In another larger multicenter study in Hong Kong, 75 patients were given the LPV/r combination as an initial therapy besides ribavirin and corticosteroids. This treatment was associated with a reduction in the overall death rate and intubation rate when compared with a matched cohort who received standard treatment (2.3% vs. 15.6% and 0% vs. 11.0%, respectively, p<0.05) and a lower rate of use of methylprednisolone at a lower mean dose [33]. The findings from the above two studies suggest that LPV/r, when combined with ribavirin, may be an effective agent against SARS.
Corticosteroids
It has been thought that the tissue damage in respiratory viral infection is caused by the vigorous systemic inflammation or cytokine storm [34] induced by early-response cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interferon-gamma (IFN-γ) [35, 36]. This exaggerated host cytokine response has been assumed to be responsible for the second immunopathological phase of SARS. Evidence from a recent report showed marked elevation of Th1 cytokine IFN-γ and of inflammatory cytokines IL-1, IL-6, and interleukin-12 (Il-12) for at least 2 weeks after disease onset. There is also significant elevation of neutrophil chemokine interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), and Th1 chemokine IFN-γ-inducible protein-10 (IP-10). Corticosteroids significantly reduce IL-8, MCP-1, and IP-10 concentrations 5–8 days after treatment. The findings confirm the activation of Th1 cell-mediated immunity and hyperinnate inflammatory response in SARS through the accumulation of monocytes/macrophages and neutrophils [37].
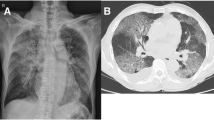
Another rationale favoring steroid usage is the necropsy evidence of infiltration by macrophages as the prominent leukocytes in the pulmonary alveoli, hemophagocytosis in the lungs, accompanied by pathological features of ARDS [38, 39], and BOOP [40]. Hemophagocytosis has been associated with cytokine dysregulation [41]. This cytokine response may be tempered by corticosteroids to prevent a fatal outcome, as suggested for ARDS of other etiologies [8, 42]. The final reason for the use of corticosteroids in SARS is that CT scans of the thorax have shown radiographic features of BOOP [43], which is a steroid-responsive condition suggestive of an immunological phenomenon [9]. Chest radiograph resolution may slightly lag behind clinical recovery, but it should not be the reason for additional doses of corticosteroids [44]. Despite a tapering down of the corticosteroids in the ensuing 2–3 weeks, the radiological opacities will usually continue to improve.
The use of corticosteroids may, nevertheless, be associated with enhancement of viral replication due to suppression of the innate immune response. One study documented a case of increased viral load following pulse methylprednisolone [25]. Another study also found that early corticosteroid treatment (<7 days of illness) was associated with a higher subsequent plasma viral load [45].
Low-dose corticosteroids, like prednisolone at 0.5–1.0 mg/kg/day, are usually used in infections and ARDS. On the other hand, pulse doses of methylprednisolone at 0.5–1.0 g/day have been widely used in SARS, especially when patients deteriorated clinically in the second week. Whether pulse corticosteroids are efficacious is unknown. Although a number of reports on corticosteroid usage in SARS have emanated from Hong Kong [46–53] and China [54–59], meaningful interpretation of these retrospective analyses is hampered by methodological limitations generated by the different types of corticosteroid dosing regimens and the time of treatment initiation during the course of disease. Despite the number of studies published, no systematic review or meta-analysis on the efficacy of corticosteroids in SARS has been available. Anecdotal cases of SARS have been successfully treated by administering methylprednisolone pulses, which correspond to the immunosuppression doses used to prevent organ rejection [52]. The same group of researchers studied the response of patients with SARS to various steroid regimens and showed that high-dose pulse methylprednisolone therapy (in the organ rejection treatment range) was more efficacious and equally safe as regimens of lower dosage [53]. Another group, however, did not find the use of pulse methylprednisolone to be associated with a better outcome [50]. Furthermore, one retrospective case series found that pulse methylprednisolone was a predictor of 30-day mortality in multivariate analysis [11]. Some groups advocated the early introduction of high-dose corticosteroids, mostly pulse methylprednisolone, in the treatment of SARS [25, 53]. Others proposed that corticosteroids should be used only judiciously in the immunopathological phase of SARS, which is towards the end of the first week and the beginning of the second week of disease. The recommended doses are lower, and pulse methylprednisolone is used whenever a patient deteriorates clinically or develops respiratory distress [51, 59]. The latter approach seems to be gaining more acceptance.
The use of corticosteroids in SARS, especially at high doses and for prolonged periods, has been accompanied by significant side effects. These include hyperglycemia, hypokalemia, hypertension, gastrointestinal hemorrhage [21, 50, 51], and, in particular, nosocomial infections [48, 50, 51, 60]. The occurrence of nosocomial infections was more prevalent in patients managed in the ICU and could manifest as pneumonia, urinary tract infection, bacteremia, and deep-seated mycosis. It is not easy to distinguish whether the recurrence of fever later in the course of SARS therapy, particularly after a period of corticosteroid treatment, is due to nosocomial infection. Anti-pseudomonal antibiotics or antifungal agents are usually indicated under such circumstances. For patients with no apparent clinical response to empirical antimicrobial therapy and in whom opportunistic infection is reasonably excluded, higher pulse methylprednisolone doses can be tried.
Avascular necrosis of bone, or osteonecrosis, is probably one the most distressing complications associated with the use of corticosteroids in SARS [61, 62]. In one recent study of 254 patients, magnetic resonance images showed evidence of subchondral osteonecrosis, mainly involving the proximal femur, in 12 (5%) patients and additional nonspecific subchondral and intramedullary bone marrow abnormalities in 77 (30%). Results of multiple logistic regression analysis confirmed the cumulative prednisolone-equivalent dose to be the most important risk factor for osteonecrosis, while joint pain was common after SARS infection and was not a useful clinical indicator of this complication [63].
Convalescent plasma
In patients who deteriorated irrevocably despite receiving pulse methylprednisolone, convalescent plasma, obtained from patients who recovered from SARS, had been used as a last resort. A cell separator operating on the plasma exchange mode is used to harvest convalescent plasma by apharesis. The experience with this kind of treatment is very limited [64]. In a small retrospective nonrandomized study of patients with progressive disease after ribavirin and pulse methylprednisolone treatment, the plasma-treated group had a shorter hospital stay and lower mortality than the group that continued treatment with pulse methylprednisolone [65]. A later study from the same group showed a higher day-22 discharge rate among patients given the plasma infusion before day 14 of illness [66].
Immunoglobulin
Another form of salvage therapy for patients who run a relentless downhill course is intravenous immunoglobulin (IVIG) [67–69] or pentaglobin infusion. IVIG is a nonspecific hyperimmune globulin, and pentaglobin is an IgM-enriched immunoglobulin preparation. A retrospective analysis of 12 patients with severe SARS demonstrated that pentaglobin is a safe and probably effective treatment in case of corticosteroid resistance [70]. Nevertheless, the use of immunoglobulin is associated with the risk of venous thrombosis.
Noninvasive positive pressure ventilation
Noninvasive positive pressure ventilation (NIPPV) is a form of ventilatory support through a tight-fitting facemask or nasal mask frequently administered in combination with continuous positive airway pressure or bi-level positive airway pressure for patients with impending respiratory failure. It has been shown that NIPPV can reduce the intubation rate, the length of ICU stay, and the 2-month mortality in patients with chronic obstructive airways disease suffering from severe community-acquired pneumonia [71]. SARS is a clinical syndrome that often progresses to varying degrees of respiratory failure. There have been anecdotal reports of the efficacy of NIPPV in SARS patients with respiratory decompensation in China and Hong Kong [49, 54, 55, 58, 72, 73]. In an early Hong Kong trial involving 20 patients with acute respiratory failure secondary to SARS, NIPPV was administered with patient isolation (single room, preferably with negative pressure), adequate airflow, full personal protective equipment, exhalation port that generates round-the-tube airflow, and interposition of a viral-bacterial filter between the mask and the exhalation port. Retrospective analysis shows that the procedure, started a mean of 9.6 days after the onset of illness in the NIPPV group, resulted in the avoidance of intubation in 70% of subjects, a shorter length of ICU stay, and a lower chest radiography score compared with the intubated group. There was no SARS infection in the healthcare workers who cared for these patients [74]. NIPPV was later banned in Hong Kong because of the fear of viral spread through air leakage around the mask with aerosol generation. However, evidence does show that NIPPV is a useful and safe treatment option for SARS patients with respiratory failure, provided it is performed under good precautions and in a suitable setting.
Invasive mechanical ventilation
When patients deteriorate or fail to improve after 1–2 days of NIPPV, or if NIPPV is contraindicated, endotracheal intubation and mechanical ventilation have to be considered. The ventilatory approach is similar to that for other causes of ARDS. The plateau pressures are kept lower than 30 cm H2O because of the tendency for barotrauma in SARS [25].
Novel treatment approaches
The present therapy of SARS is less than satisfactory, and much effort has been expended in looking for novel treatment modalities. It is well known that viral infections can stimulate the production of interferons (IFNs) in the early phase of the human hereditary immune response. The cytopathic effects of SARS-CoV have shown to be completely inhibited in vitro by IFN β [75], by IFN β-1b, IFN α-n1, IFN α-n3, human leukocyte IFN-α [76], and by IFN β-1a [77]. Synergistic inhibition of the replication of SARS-CoV has been achieved by the combination of IFN-β plus IFN-γ in animal cells [78], and by IFN-β plus ribavirin in both animal and human cells [79]. In vitro data from Hong Kong has shown that type I interferons (IFN-α and -β) can induce potent inhibition of the SARS-CoV, while type II interferons (IFN-γ) cannot [80]. An uncontrolled trial found that IFN alfacon-1 plus corticosteroids improved oxygen saturation, hastened radiographic resolution of lung opacities, and lowered the levels of creatine kinase [81]. In an animal study, pegylated IFN-α was associated with significantly reduced viral replication, excretion, and expression in lung tissue when administered prophylactically to cynomolgus macaques before experimental exposure to SARS-CoV. Postexposure treatment resulted in intermediate outcomes [82]. The above findings provide the basis for further pursuit of the role of interferons in the treatment and prophylaxis of SARS.
There has been some evidence indicating that the SARS-CoV can enter human cells through binding of the viral S1 protein to the angiotensin-converting enzyme-2 receptor [83], though the clinical significance of this finding was not confirmed by a recent study [84]. A high-affinity human monoclonal antibody, called 80R, has been demonstrated to have strong neutralizing activity in vitro and in vivo against the S1 protein and has been hypothesized to be a useful agent for SARS treatment and prophylaxis [85]. However, it should be noted that enhanced disease with other coronaviruses, including SARS-CoV, may occur with S1-specific antibodies.
There are several other potential treatment options that may eventually prove to be valuable anti-SARS agents after further investigation. The active component of liquorice root, glycyrrhizin, shows significant viral inhibition in vitro [17]. The SARS-CoV also exhibits in vitro susceptibility to baicalin, a chemical compound derived from the herb scute (huangqin) [86]. Nelfinavir was found to inhibit the cytopathic effect induced by SARS-CoV infection and decrease the production of virions from Vero cells [87]. More agents on the horizon are now under intensive research, including small interfering RNAs [88], small-molecule inhibitors identified by chemical genetics [89], target-designed proteinase inhibitors [90], existing drugs used for treatment of HIV, psychotic disorders, and parasitic infections [91, 92], tumor necrosis factor-alpha inhibitor [93], nitric oxide [94, 95], and aminopeptidase N inhibitors [96].
Traditional Chinese medicine
During the SARS outbreak, traditional Chinese medicine (TCM) was used extensively in China. A large experience has been accumulated in the TCM literature [97–99]. As compared with Western methods of treatment, integrated TCM and Western medicine treatment has been shown to be more effective in improving clinical symptoms, shortening the course of illness, clearing up lung inflammation, preventing rebound of fever, and reducing the duration of corticosteroid usage. Selected cases in Hong Kong also were treated with TCM as an adjuvant therapy, especially in the convalescent period. The benefits are difficult to establish, due to the heterogeneous type and amount of the concoction constituents, the different phases of disease when therapy was started, the diverse clinical condition of the patients, and the variable length of treatment. In an investigation on the relationships between the hazard for death and that for cure, a cure-death hazard plot was developed for the case-fatality rates in Hong Kong, Singapore, and Beijing. The results showed a significant difference in the case-fatality rates between Beijing and other areas. It is postulated that the phenomenon may be due to the difference in treatment methods, and it is possible that combining TCM with Western medicine gives better results than the latter alone in the treatment of SARS [100]. This aspect of anti-SARS therapy certainly warrants more investigations in the future.
Treatment protocols
A number of treatment protocols were reported in Hong Kong during the SARS outbreak. They were invariably composed of antibiotics, a combination of doses and route of administration for ribavirin, a step-down course of corticosteroids over a few weeks, and possibly pulse methylprednisolone rescue in cases of clinical worsening of patients [49–53]. It was found, in one report, that adherence to a standard treatment protocol resulted in overall satisfactory outcomes [101].
Treatment guidelines, intended for use should SARS return, were worked out through consensus among experts in Hong Kong at the end of 2003 [102]. The protocol has the format of a placebo-controlled double-blinded randomized trial. Patients who fulfill the WHO clinical case definition of SARS [103] are prescribed broad-spectrum antibiotics (3rd/4th-generation cephalosporin plus macrolide if not penicillin allergic; antipneumococcal quinolone for penicillin-allergic patients) and supportive care. Upon laboratory confirmation of SARS according to the WHO definitions and if the patient's condition is stable, informed consent is obtained and the patient is randomized to one of two arms: the antiviral arm or the placebo arm. Patients assigned to the antiviral arm receive the ribavirin and lopinavir/ritonavir combination. Ribavirin is started at a 2.4 g oral loading dose followed by 1.2 g orally for a total of 10 days. The lopinavir/ritonavir combination is administered as three tablets b.i.d. orally (each tablet contains lopinavir 400 mg and ritonavir 100 mg) for a total of 10 days. As a rescue measure, when patients in either arm have features of acute lung injury defined by a PaO2/FIO2 ratio of between 26.7 and 40 kPa (200–300 mm Hg), they are given either (i) prednisolone 1.0–1.5 mg/kg/day for 5 days or more, followed by tapering down of the dosage with clinical improvement every 5 days by 0.5 mg/kg decrements until off, or (ii) methylprednisolone 3 mg/kg/day intravenously for 5 days and tapered by 1 mg/kg every 5 days (further stepping down can be by oral prednisolone) for a total of 2 weeks. In patients with underlying cardiac or respiratory condition, initiation of NIPPV can be considered. If patients develop critical SARS as defined by a PaO2/FIO2 ratio of <26.7 kPa (200 mm Hg) and progressive deterioration of chest radiograph [104], NIPPV or invasive ventilation must be considered. Pulse steroids can be used at the discretion of the clinician. The suggested regimen is methylprednisolone at 0.5 mg per day intravenously for 3 days, followed by a tapering course at 3 mg/kg/day. The cumulative dose should preferably not exceed 2 g.
Similar consensus treatment guidelines, using ribavirin and corticosteroids, were promulgated in China in November 2003 [105]. Other centers in the world, like Canada and Singapore, have not been as liberal in the use of ribavirin and corticosteroids as in Hong Kong, but interestingly, their patients have had quite similar outcomes [106, 107]. In the USA, where supportive therapy alone has been used to treat SARS patients, the case-fatality rate is 0%. However, direct comparison is not possible since only 8 of 47 probable cases and none of the 162 suspected cases had virological evidence of SARS-CoV infection [108].
Conclusion
Ribavirin and corticosteroids were the cornerstones of treatment during the SARS outbreak. Ribavirin has shown a lack of efficacy in a number of studies. The usefulness of corticosteroids has been reported in some uncontrolled trials, but further investigations are required. The antiretroviral formulation of lopinavir/ritonavir, when used with ribavirin, was found to be associated with clinical benefits. The survival of patients who deteriorate despite pulse methylprednisolone therapy may be improved by the use of convalescent plasma or immunoglobulins like pentaglobin. NIPPV, when performed in an appropriate setting under adequate precautions, has proved to be effective in patients who develop respiratory failure. Interferons and other novel agents are now under active investigation and may considerably strengthen the anti-SARS armamentarium in the future. TCM is yet an untapped wealth of knowledge and can become an important partner to the conventional treatment strategy. Finally, the optimal treatment regimen for SARS remains elusive. Randomized controlled treatment trials should be done, especially through international collaboration, to discover the best form of therapy.
References
Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Nicholls J, Yee WKS, Yan WW, Cheung MT, Cheng VCC, Chan KH, Tsang DNC, Yung RWH, Ng TK, Yuen KY, SARS Study Group (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, sSARS Working Group (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966
Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976
World Health Organization (2004) Cumulative number of reported probable cases, http://www.who.int/csr/sars/country/table2004_04_21/en/. Cited 16 February 2005
Falsey AR, Walsh EE (2003) Novel coronavirus and severe acute respiratory syndrome. Lancet 361:1312–1313
Shi J, Wei Z, Song J (2004) Dissection study on the severe acute respiratory syndrome 3C-like protease reveals the critical role of the extra domain in dimerization of the enzyme: defining the extra domain as a new target for design of highly specific protease inhibitors. J Biol Chem 279:24765–24773
Liu Z, Huang C, Fan K, Wei P, Chen H, Liu S, Pei J, Shi L, Li B, Yang K, Liu Y, Lai L (2005) Virtual screening of novel noncovalent inhibitors for SARS-CoV 3C-like proteinase. J Chem Inf Model 45:10–17
Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 280:159–165
Epler GR (2001) Bronchiolitis obliterans organizing pneumonia. Arch Intern Med 161:158–164
Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk HD, Doecke WD, Falke KJ, Gerlach H (2003) Immunologic and hemodynamic effects of “low dose” hydrocortisone in septic shock. Am J Resp Crit Care Med 167:512–520
Tsang OTY, Chau TN, Choi KW, Tso EYK, Lim W, Chiu MC, Tong WL, Lee PO, Lam BHS, Ng TK, Lai JY, Yu WC, Lai ST (2003) Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerg Infect Dis 9:1381–1387
Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, Kao RYT, Poon LLM, Wong CLP, Guan Y, Peiris JSM, Yuen KY, on behalf of the HKU/UCH SARS Study Group (2004) Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59:252–256
Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK (1972) Broad-spectrum antiviral activity of virazole: 1-beta-d-ribofuranosyl-1, 2, 4-triazole-3-carboxamide. Science 177:705–706
Cameron CE, Castro C (2001) The mechanism of action of ribavirin: lethal mutagenesis of RNA virus mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis 12:261–272
Huggins JW (2003) Severe acute respiratory syndrome (SARS) and coronavirus testing–United States. Morb Mortal Wkly Rep 52:297–302
Health Canada (2003) Management of severe acute respiratory syndrome (SARS) in adults: interim guidance for health providers. http://www.hc.sc.gc.ca/. Cited 16 February 2005
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW (2003) Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 3361:2045–2046
Leong HN, Ang B, Earnest A, Teoh C, Xu W, Leo YS (2004) Investigational use of ribavirin in the treatment of severe acute respiratory syndrome, Singapore, 2003. Trop Med Int Health 9:923–927
Stroher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, Jones SM, Feldmann H (2004) Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J Infect Dis 189:1164–1167
Booth CM, Matukas LM, Tomlinsom GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS (2003) Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801–2809
Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, Lee PO, Ng TK, Ng WF, Lee KC, Lam W, Yu WC, Lai JY, Lai ST, Princess Margaret Hospital SARS Study Group (2003) Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med 139:715–723
Cyranoski D (2003) Critics slam treatment for SARS as ineffective and perhaps dangerous. Nature 423:4
Wenzel RP, Edmond MB (2003) Managing SARS amidst uncertainty. N Engl J Med 348:1947–1948
Knowles SR, Phillips EJ, Dresser L, Matukas L (2003) Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome. Clin Infect Dis 37:1139–1142
Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY, HKU/UCH SARS Study Group (2003) Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361:1767–1772
van Vonderen MGA, Bos JC, Prins JM, Wertheim-van Dillen P, Speelman P (2003) Ribavirin in the treatment of severe acute respiratory syndrome (SARS). Neth J Med 61:238–241
Zhaori G (2003) Antiviral treatment for SARS: can we draw any conclusion? Can Med Assoc J 169:1165–1166
Hurst M, Faulds D (2000) Lopinavir. Drugs 60:1371–1379
Cvetkovic RS, Goa KL (2003) Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs 63:769–802
Leung WK, To KF, Chan PKS, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ (2003) Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125:1011–1017
Kwan ACP, Chau TN, Tong WL, Tsang OTY, Tso EYK, Chiu MC, Yu WC, Lai TST (2005) Severe acute respiratory syndrome (SARS) related diarrhoea. J Gastroenterol Hepatol 20:606–610
Lee PO, Tsui PT, Tsang TY, Chau TN, Kwan CP, Yu WC, Lai ST, Princess Margaret Hospital SARS Study Group (2005) Severe acute respiratory syndrome: clinical features. In: Schmidt A, Wolff MH, Weber O (eds) Coronaviruses with special emphasis on first insights concerning SARS. Birkhäuser Verlag, Basel, pp 71–85
Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, Tse MW, Que TL, Peiris JS, Sung J, Wong VC, Yuen KY (2003) Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J 9:399–406
Lee N, Sung J (2003) The use of corticosteroids in SARS. N Engl J Med 348:2034–2035
Van Reeth K, Van Gucht S, Pensaert M (2002) Correlations between lung proinflammatory cytokine levels, virus replication, and disease after swine influenza challenge of vaccination-immune pigs. Viral Immunol 15:583–594
Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS (2002) Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831–1837
Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ (2004) Plasma inflammatory cytokines in severe acute respiratory syndrome. Clin Exp Immunol 136:95–103
Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, Yan KW, Chan KH, Tsang NC, Guan Y, Yuen KY, Peiris JS (2003) Lung pathology of fatal severe acute respiratory syndrome. Lancet 361:1773–1778
Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, Selbs E, McEvoy CP, Hayden CD, Fukuoka J, Taubenberger JK, Travis WD (2003) Lung pathology in severe acute respiratory syndrome (SARS): a study of 8 autopsy cases in Singapore. Hum Pathol 34:743–748
Tse GM, To KF, Chan PK, Lo AW, Ng KC, Wu A, Lee N, Wong HC, Mak SM, Chan KF, Hui DS, Sung JJ, Ng HK (2004) Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol 57:260–265
Fishman DN (2000) Hemophagocytic syndrome and infection. Emerg Infect Dis 6:60–68
Lai KN, Leung JC, Metz CN, Lai FM, Bucala R, Lan HY (2003) Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J Pathol 199:496–508
Wong KT, Antonio GE, Hui DS, Lee N, Yuen EH, Wu A, Leung CB, Rainer TH, Cameron P, Chung SS, Sung JJ, Ahuja AT (2003) Thin scan CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology 228:395–400
Yao W, Chen Y, Zhang L, Wang X, Sun Y, Sun W, Han J, Zhang F, Zheng Y, Sun B, He B, Zhao M (2004) Chest X-ray changes in severe acute respiratory syndrome cases after discontinuation of glucocorticosteroids treatment. Chin Med J 117:143–144
Lee N, Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM (2004) Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 31:304–309
Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN (2003) A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1977–1985
Lee N, Hui DS, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1986–1994
Tsui PT, Kwok ML, Yuen H, Lai ST (2003) Severe acute respiratory syndrome: clinical outcomes and prognostic correlates. Emerg Infect Dis 9:1064–1069
So LK, Lau AC, Yam LY, Cheung TM, Poon E, Yung RW, Yuen KY (2003) Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet 361:1615–1617
Chan JWM, Ng KC, Chan YH, Mok TYW, WHO, Lee S, Chu SYY, Law WL, Lee MP, Li PCK (2003) Short-term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 58:686–689
Sung JJY, Wu A, Joynt GM, Yuen KY, Lee N, Chan PKS, Cockram CS, Ahuja AT, Yu LM, Wong VW, Hui DSC (2003) Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax 59:414–420
Tsang KW, Lam WK (2003) Management of severe acute respiratory syndrome – the Hong Kong University experience. Am J Resp Crit Care Med 168:417–424
Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B, Wong PC, Li PC, Ho PL, Lam WK, Ng KC, Ip MS, Lai KN, Chan-Yeung M, Tsang KW (2003) High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Resp Crit Care Med 168:1449–1456
Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, Yin Z, Huang S, Deng Z, Wei M, Xiong J, Hawkey PM (2003) Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol 52:715–720
Wu W, Wang J, Liu P, Chen W, Yin S, Jiang S, Yan L, Zhan J, Chen X, Li J, Huang Z, Huang H (2003) A hospital outbreak of severe acute respiratory syndrome in Guangzhou, China. Chin Med J 116:811–818
Zheng ZG, Zhong NS (2003) The use of corticosteroids in management of critical SARS. Chin Med J 42:676–677
Zhong NS, Zeng GQ (2003) Our strategies for fighting severe acute respiratory syndrome (SARS). Am J Resp Crit Care Med 168:7–9
Li N, Ma J, Nie L, Li H, Que C, Gao Z, Wang G, Xu X, Lu H, Wang G (2003) Retrospective analysis of the corticosteroids treatment on severe acute respiratory syndrome (SARS) Beijing Daxue Xuebao 35(Suppl):16–18
Sun H-Y, Fang C-T, Wang J-T, Chen Y-C, Chang S-C (2003) Treatment of severe acute respiratory syndrome in health-care workers. Lancet 362:2025–2026
Wang H, Ding Y, Li X, Yang L, Zhang W, Kang W (2003) Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N Engl J Med 349:507–508
Hong N, Du XK (2004) Avascular necrosis of bone in severe acute respiratory syndrome. Clin Radiol 59:602–608
Chan CW, Chiu WK, Chan CC, Chow EY, Cheung HM, Ip PL (2004) Osteonecrosis in children with severe acute respiratory syndrome. Pediatr Infect Dis J 23:888–890
Griffith JF, Antonio GE, Kumta SM, Hui DSC, Wong JKT, Joynt GM, Wu AKL, Cheung AY, Chiu KH, Chan KM, Leung PC, Ahuja AT (2005) Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology 235:168–175
Wong VW, Dai D, Wu AK, Sung JJ (2003) Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J 9:199–201
Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, Ng MH, Chan P, Cheng G, Sung JJ (2004) Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infection 10:676–678
Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G (2005) Use of convalescent plasma in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 24:44–46
Ali MB (2003) Treating severe acute respiratory syndrome with hyperimmune globulins. Hong Kong Med J 9:391–392
Chiang CH, Chen HM, Shih JF, Su WJ, Perng RP (2003) Management of hospital-acquired severe acute respiratory syndrome with different disease spectrum. J Chin Med Assoc 66:328–338
Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, Kor AC, Yap WS, Chelliah YR, Lai YC, Goh SK (2003) Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 290:374–380
Ho JC, Wu AY, Lam B, Ooi GC, Khong PL, Ho PL, Chan-Yeung M, Zhong NS, Ko C, Lam WK, Tsang KW (2004) Pentaglobin in steroid-resistant severe acute respiratory syndrome. Int J Tuberc Lung Dis 8:1173–1179
Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G (1999) Acute respiratory failure in patients with severe community-acquired pneumonia. Am J Resp Crit Care Med 160:1585–1591
Luo D, Qian SC (2003) SARS treatment: experience from a team in Guangdong, China. Chin Med J 116:838–839
Yam LYC, Chen RC, Zhong NS (2003) SARS: ventilatory and intensive care. Respirology 8(Suppl):S31–S35
Cheung TMT, Yam LYC, So LKY, Lau ACW, Poon E, Kong BMH, Yung RWH (2004) Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest 126:845–850
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW (2003) Treatment of SARS with human interferons. Lancet 362:748
Tan EL, Ooi EE, Lin CY, Tan HC, Ling AE, Lim B, Stanton LW (2004) Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis 10:581–586
Hensley LE, Fritz EA, Jahrling PB, Karp CL, Huggins JW, Geisbert TW (2004) Interferon-β1a and SARS coronavirus replication. Emerg Infect Dis 10:317–319
Sainz B Jr, Mossel EC, Peters CJ, Garry RF (2004) Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology 329:11–17
Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J Jr (2005) Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun 326:905–908
Zheng B, He ML, Wong KL, Lum CT, Poon LL, Peng Y, Guan Y, Lin MC, Kung HF (2004) Potent inhibition of SARS-associated coronavirus (SCoV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J Interferon Cytokine Res 24:388–390
Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Pham DH, Deif H, LaMere EA, Chang M, Kain KC, Farcas GA, Ferguson P, Latchford M, Levy G, Dennis JW, Lai EK, Fish EN (2003) Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA 290:3222–3228
Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, van Amerongen G, van Riel D, de Jong T, Itamura S, Chan KH, Tashiro M, Osterhaus AD (2004) Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 10:290–293
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454
Chiu RW, Tang NL, Hui DS, Chung GT, Chim SS, Chan KC, Sung YM, Chan LY, Tong YK, Lee WS, Chan PK, Lo YM (2004) ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clin Chem 50:1683–1686
Sui J, Li W, Murakami A, Tamin A, Matthews LJ, Wong SK, Moore MJ, Tallarico AS, Olurinde M, Choe H, Anderson LJ, Bellini WJ, Farzan M, Marasco WA (2004) Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci U S A 101:2536–2541
Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY (2004) In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 31:69–75
Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J, Rabenau H, Doerr HW, Hunsmann G, Otaka A, Tamamura H, Fujii N, Yamamoto N (2004) HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun 318:719–725
He ML, Zheng B, Peng Y, Peiris JS, Poon LL, Yuen KY, Lin MC, Kung HF, Guan Y (2003) Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA 290:2665–2666
Kao RY, Tsui WHW, Lee TSW, Tanner JA, Watt RM, Huang J-D, Hu L, Chen G, Chen Z, Zhang L, He T, Chan K-H, Tse H, To APC, Ng LWY, Wong BCW, Tsoi H-W, Yang D, Ho DD, Yuen K-Y (2004) Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem Biol 11:1293–1299
Zhang XW, Yap YL (2004) Exploring the binding mechanism of the main proteinase in SARS-associated coronavirus and its implication to anti-SARS drug design. Bioorg Med Chem 12:2219–2223
Zhang XW, Yap YL (2004) Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg Med Chem 12:2517–2521
Chen XP, Cao Y (2004) Consideration of highly active antiretroviral therapy in the prevention and treatment of severe acute respiratory syndrome. Clin Infect Dis 38:1030–1032
Tobinick E (2004) TNF-alpha inhibition for potential therapeutic modulation of SARS coronavirus infection. Curr Med Res Opin 20:39–40
Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M (2004) Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis 8:223–226
Akerstrom S, Mousavi-Jazi M, Klingstrom J, Leijon M, Lundkvist A, Mirazimi A (2005) Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol 79:1966–1969
Kontoyiannis DP, Pasqualini R, Arap W (2003) Aminopeptidase N inhibitors and SARS. Lancet 361:1558
Lin L, Han Y, Yang ZM (2003) Clinical observation on 103 patients of severe acute respiratory syndrome treated by integrative traditional Chinese and Western medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 23:409–413
Zhang RL, Jiao Q, Wang BG (2003) Controlled clinical study on 49 patients of SARS treated by integrative Chinese and Western medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 23:654–657
Li J, Li SD, Du N (2004) Clinical study on treatment of severe acute respiratory syndrome with integrative Chinese and Western medicine approach. Zhongguo Zhong Xi Yi Jie He Za Zhi 24:28–31
Chen Z, Nakamura T (2004) Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res 18:592–594
Lau AC, So LK, Miu FP, Yung RW, Poon E, Cheung TM, Yam LY (2004) Outcome of coronavirus-associated severe acute respiratory syndrome using a standard treatment protocol. Respirology 9:173–183
Hong Kong Hospital Authority (2004) Treatment guidelines for adult patients with SARS. Updated 11 February 2004. http://ha.home/ho/pa/UpdatedTreatmentGuidelines040416.doc. Cited 16 February 2005
World Health Organization (2004) WHO guidelines for the global surveillance of severe acute respiratory syndrome (SARS). Updated recommendations, October 2004. http://www.who.int/csr/resources/publications/en/WHO_CDS_CSR_ARO_2004_1.pdf. Cited 16 February 2005
Tsang KW, Zhong NS (2003) SARS: pharmacotherapy. Respirology 8(Suppl):S25–S30
Zhong N, Ding Y, Mao Y, Wang Q, Wang G, Wang D, Cong Y, Li Q, Liu Y, Ruan L, Chen B, Du X, Yang Y, Zhang Z, Zhang X, Lin J, Zheng J, Zhu Q, Ni D, Xi X, Zeng G, Ma D, Wang C, Wang W, Wang B, Wang J, Liu D, Li X, Liu X, Chen J, Chen R, Min F, Yang P, Zhang Y, Luo H, Lang Z, Hu Y, Ni A, Cao W, Lei J, Wang S, Wang Y, Tong X, Liu W, Zhu M, Zhang Y, Zhang Z, Zhang X, Li X, Chen W, Xhen X, Lin L, Luo Y, Zhong J, Weng W, Peng S, Pan Z, Wang Y. Wang R, Zuo J, Liu B, Zhang N, Zhang J, Zhang B, Zhang Z, Wang W, Chen L, Zhou P, Luo Y, Jiang L, Chao E, Guo L, Tan X, Pan J, Chinese Medical Association, China Association of Chinese Medicine (2003) Consensus for the management of severe acute respiratory syndrome. Chin Med J 116:1603–1635
Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ, Canadian Severe Acute Respiratory Syndrome Study Team (2003) Identification of severe acute respiratory syndrome in Canada. N Engl J Med 348:1995–2005
Hsu LY, Lee CC, Green JA, Ang B, Paton NI, Lee L, Villacian JS, Lim PL, Earnest A, Leo YS (2003) Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis 9:713–717
Anonymous (2003) Update: severe acute respiratory syndrome—United States, 2003. MMWR Morb Mortal Wkly Rep 52:616
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, S.T. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis 24, 583–591 (2005). https://doi.org/10.1007/s10096-005-0004-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-005-0004-z