Abstract
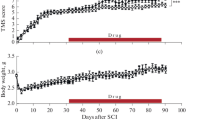
Inosine, a purine nucleoside, is one of the novel substances, which can preserve the neuronal and glial viability and stimulate intact neurons to extend axons. We, herein, evaluated the effect of oral inosine treatment on spinal cord injury (SCI) recovery by means of locomotor and bladder function, quantification of neurons and spinal cord tissue sparing. Rats after compression SCI were divided into groups—SCI-Aqua and SCI-Inosine (daily application of aqua for injection or inosine)—locomotion of hind limbs (BBB score) and urinary bladder function were evaluated from day 1 to 28 after SCI. The neuronal profile was determined by immunohistochemistry with NeuN antibodies and tissue sparing by Luxol fast blue staining method. SCI affected the functional movement of hind limbs in both groups with gradual improvement (increased BBB score) during survival. However, we found a significant difference in BBB score and recovery of bladder function between SCI-Aqua and SCI-Inosine groups during the second week of survival following SCI. In addition, the number of NeuN positive cells and percentage of tissue sparing was also significantly higher in SCI-Inosine group when compared with the SCI-Aqua group. Daily oral administration of inosine after SCI throughout the survival was beneficial for locomotion and micturition, neuronal survival and tissue sparing. This indicates that inosine may represent one of the co-stimulatory factors for treatment strategies to promote neuronal plasticity after SCI.





Similar content being viewed by others
References
McKee WM (1990) Spinal trauma in dogs and cats: a review of 51 cases. Vet Rec 26(12):285–289
Coates JR (2000) Intervertebral disk disease. Vet Clin N Am Small Anim Pract 30(1):77–110
Webb AA, Ngan S, Fowler D (2010) Spinal cord injury II: prognostic indicators, standards of care and clinical trials. Can Vet J 51(6):598–604
Park EH, White GA, Tieber LM (2012) Mechanisms of injury and emergency care of acute spinal cord injury in dogs and cats. J Vet Emerg Crit Care 22(2):160–178
Mautes AEM, Weinzierl MR, Donovan F, Noble LJ (2000) Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther 80(7):673–687
Sharp NJH, Wheeler SJ (2005) Small animal spinal disorders: diagnosis and treatment, 2nd edn. Elsevier, Amsterdam
Fawcet W (2006) Overcoming inhibition in the damaged spinal cord. J Neurotrauma 23(3–4):371–383
Fawcet W, Asher RA (1999) The glial scar and central nervous system repair. Brian Res Bull 49(6):377–391
Schwab ME (2004) Nogo and axon regeneration. Curr Opin Neurobiol 14(1):118–124
Hollis ER, Tuszynski MH (2011) Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics 8(4):694–703
Nagahara AH, Mateling M, Kovacs I et al (2013) Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci 33(39):15596–15602
Gomez G, Sitkovsky MV (2003) Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood 102(13):4472–4478
Liu F, You SW, Yao LP et al (2006) Secondary degeneration reduced by inosine after spinal cord injury in rats. SpinalCord 44(7):421–426
Bohnert DM, Purvines S, Shapiro S, Borgens RB (2007) Simultaneous application of two neurotrophic factors after spinal cord injury. J Neurotrauma 24(5):846–863
Conta AC, Stelzner DJ (2008) Immunomodulatory effect of the purine nucleoside inosine following spinal cord contusion injury in rat. Spinal Cord 46(1):39–44
Petrausch B, Tabibiazar R, Roser T et al (2000) A purine-sensitive pathway regulates multiple genes involved in axon regeneration in goldfish retinal ganglion cells. J Neurosci 20(21):8031–8041
Zai L, Ferrari Ch, Subbaiah S et al (2009) Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci 29(25):8187–8197
Zai L, Ferrari C, Dice C et al (2011) Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. J Neurosci 31(16):5977–5988
Jurkowitz MS, Litsky ML, Browning MJ, Hohl ChM (1998) Adenosine, inosine, and guanosine protect glial cells during glucose deprivation and mitochondrial inhibition: correlation between protection and ATP preservation. J Neurochem 71(2):535–548
Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS (1999) Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res 821(2):426–432
Wu Z, Liu F, Wang Y et al (2008) Reduced cell death by inosine pretreatment after photochemically induced cerebral ischemia in adult rats. Prog Nat Sci 18(12):1513–1518
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Vanicky I, Urdzikova L, Saganova K, Cizkova D et al (2001) Simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma 18(12):1399–1407
Wakai A, Winter DC, Street JT et al (2001) Inosine attenuates tourniquet-induced skeletal muscle reperfusion injury. J Surg Res 99(2):311–315
Guerrero AL, Gutiérrez F, Iglesias F et al (2011) Serum uric acid levels in multiple sclerosis patients inversely correlate with disability. Neurol Sci 32(2):347–350
Sotgiu S, Pugliatti M, Sanna A et al (2002) Serum uric acid and multiple sclerosis. Neurol Sci 23(4):183–188
Markowitz CE, Spitsin S, Zimmerman V et al (2009) The treatment of multiple sclerosis with inosine. J Altern Complement Med 15(6):619–625
Liu F, Yao L, Yuan J et al (2009) Protective effects of inosine on urinary bladder function in rats with partial bladder outlet obstruction. Urology 73:1417–1422
Lee YS, Lin ChY, Jiang HH et al (2013) Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci 33(26):10591–10606
Benowitz LI, Goldberg DE, Madsen JR et al (1999) Inosine stimulates extensive axon collateral growth in the rat corticospinal tract after injury. Proc Natl Acad Sci USA 96(23):13486–13490
Chen P, Goldberg DE, Kolb B et al (2002) Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci USA 99(13):9031–9036
Li S, Juan YS, Kogan BA et al (2009) Effects of inosine on response to in vitro hypoxia in absence of substrate on bladder dysfunction in adult rats. Urology 73(3):661–664
Mubagwa K, Flameng W (2001) Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res 52:25–39
Yu W, Zacharia LC, Jackson EK, Apodaca G (2006) Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol 291:C254–C265
Jin X et al (1997) Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest 100:2849–2857
Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770
Hasko G, Cronstein BN (2004) Immunomodulatory and neuroprotective effects of inosine. Trends Pharm Sci 25(3):152–157
Hasko G, Kuhel DG, Nemeth ZH et al (2000) Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol 164:1013–1019
Lam-Tse WK, Lernmark A, Drexhage HA (2002) Animal models of endocrine/organ-specific autoimmune diseases: do they really help us to understand human autoimmunity? Springer Semin Immunopathol 24:297–321
Dowdall JF, Winter DC et al (2002) Inosine modulates gut barrier dysfunction and end organ damage in a model of ischemia-reperfusion injury. J Surg Res 108:61–68
Garcia Soriano F, Liaudet L, Marton A et al (2001) Inosine improves gut permeability and vascular reactivity in endotoxic shock. Crit Care Med 29:703–708
Yoles E, Hauben E, Palgi O et al (2001) Protective autoimmunity is a physiological response to CNS trauma. J Neurosci 21(11):3740–3748
Lorber B, Howe ML, Benowitz LI, Irwin N (2009) Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat Neurosci 12:1407–1414. doi:10.1038/nn.2414
Politi V (2002) Uridine toxicity. http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0182-05-tab-02-vol134-web.pdf
Acknowledgments
This work was supported by the financial support from the Scientific Grant Agency of Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak academy of Sciences (VEGA no. 1/2945/12, VEGA no. 2/0169/13) and the Slovak Research and Development Agency (APVV no. 0472/11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuricova, M., Ledecky, V., Liptak, T. et al. Oral administration of inosine promotes recovery after experimental spinal cord injury in rat. Neurol Sci 35, 1785–1791 (2014). https://doi.org/10.1007/s10072-014-1840-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1840-3