Abstract
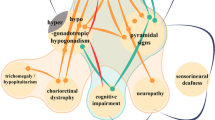
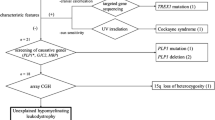
Leukodystrophies are a heterogeneous group of disorders associated with abnormal central nervous system white matter. The clinical features invariably include upper motor neuron signs and developmental regression with or without other neurological manifestations. The objective of this study was to characterize clinically and genetically a new form of childhood-onset leukodystrophy with ataxia and tremor. We recruited seven French-Canadian cases belonging to five families affected by an unknown form of childhood-onset leukodystrophy. Genome-wide scans (GWS) were performed using the Illumina Hap310 or Hap610 Bead Chip to identify regions of shared homozygosity that were further studied for linkage with STS markers. All cases presented between the ages of 1 and 5 years with spasticity along with other upper motor neuron signs, prominent postural tremor, and cerebellar signs. Though motor regression is a constant feature, cognitive functions are relatively preserved, even late in the course of the disease. The higher frequency of founder diseases in the French-Canadian population and the segregation in pedigrees are suggestive of a recessive mode of inheritance. By homozygosity mapping, we established linkage to a 12.6-Mb SNP-haplotyped region on chromosome 10q22.3–10q23.31 (maximum LOD score: 5.47). We describe an autosomal recessive childhood-onset leukodystrophy with ataxia and tremor mapping to a 12.6 Mb interval on chromosome 10q22.3–10q23.31. Identification of the mutated gene will allow precise diagnosis and genetic counseling and shed light on how its perturbed function leads to white matter abnormalities.



Similar content being viewed by others
References
Schiffmann R, van der Knaap MS (2004) The latest on leukodystrophies. Curr Opin Neurol 17:187–192
Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 27:117–120
Cannizzaro LA, Chen YQ, Rafi MA, Wenger DA (1994) Regional mapping of the human galactocerebrosidase gene (GALC) to 14q31 by in situ hybridization. Cytogenet Cell Genet 66:244–245
Gieselmann V, Polten A, Kreysing J, von Figura K (1994) Molecular genetics of metachromatic leukodystrophy. J Inherit Metab Dis 17:500–509
Henseler M, Klein A, Reber M, Vanier MT, Landrieu P, Sandhoff K (1996) Analysis of a splice-site mutation in the sap-precursor gene of a patient with metachromatic leukodystrophy. Am J Hum Genet 58:65–74
Inoue K (2005) PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics 6:1–16
Kaul R, Gao GP, Balamurugan K, Matalon R (1993) Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat Genet 5:118–123
Leegwater PA, Yuan BQ, van der Steen J, Mulders J, Konst AA, Boor PK, Mejaski-Bosnjak V, van der Maarel SM, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS (2001) Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet 68:831–838
Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P (1993) Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361:726–730
Orcesi S, La PR, Fazzi E (2009) Aicardi-Goutieres syndrome. Br Med Bull 89:183–201
Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, Nurnberg P, Hubner C, Weschke B, Gartner J (2004) Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet 75:251–260
Laberge AM, Michaud J, Richter A, Lemyre E, Lambert M, Brais B, Mitchell GA (2005) Population history and its impact on medical genetics in Quebec. Clin Genet 68:287–301
Sobel E, Sengul H, Weeks DE (2001) Multipoint estimation of identity-by-descent probabilities at arbitrary positions among marker loci on general pedigrees. Hum Hered 52:121–131
Mukhopadhyay N, Almasy L, Schroeder M, Mulvihill WP, Weeks DE (2005) Mega2: data-handling for facilitating genetic linkage and association analyses. Bioinformatics 21:2556–2557
Dupre N, Gros-Louis F, Chrestian N, Verreault S, Brunet D, de Verteuil D, Brais B, Bouchard JP, Rouleau GA (2007) Clinical and genetic study of autosomal recessive cerebellar ataxia type 1. Ann Neurol 62:93–98
Duquette A, Roddier K, McNabb-Baltar J, Gosselin I, St-Denis A, Dicaire MJ, Loisel L, Labuda D, Marchand L, Mathieu J, Bouchard JP, Brais B (2005) Mutations in senataxin responsible for Quebec cluster of ataxia with neuropathy. Ann Neurol 57:408–414
Gosselin I, Thiffault I, Tetreault M, Chau V, Dicaire MJ, Loisel L, Emond M, Senderek J, Mathieu J, Dupre N, Vanasse M, Puymirat J, Brais B (2008) Founder SH3TC2 mutations are responsible for a CMT4C French-Canadians cluster. Neuromuscul Disord 18:483–492
Christomanou H, Chabas A, Pampols T, Guardiola A (1989) Activator protein deficient Gaucher's disease. A second patient with the newly identified lipid storage disorder. Klin Wochenschr 67:999–1003
Harzer K, Paton BC, Poulos A, Kustermann-Kuhn B, Roggendorf W, Grisar T, Popp M (1989) Sphingolipid activator protein deficiency in a 16-week-old atypical Gaucher disease patient and his fetal sibling: biochemical signs of combined sphingolipidoses. Eur J Pediatr 149:31–39
Kretz KA, Carson GS, Morimoto S, Kishimoto Y, Fluharty AL, O'Brien JS (1990) Characterization of a mutation in a family with saposin B deficiency: a glycosylation site defect. Proc Natl Acad Sci USA 87:2541–2544
Schnabel D, Schroder M, Furst W, Klein A, Hurwitz R, Zenk T, Weber J, Harzer K, Paton BC, Poulos A (1992) Simultaneous deficiency of sphingolipid activator proteins 1 and 2 is caused by a mutation in the initiation codon of their common gene. J Biol Chem 267:3312–3315
Spiegel R, Bach G, Sury V, Mengistu G, Meidan B, Shalev S, Shneor Y, Mandel H, Zeigler M (2005) A mutation in the saposin A coding region of the prosaposin gene in an infant presenting as Krabbe disease: first report of saposin A deficiency in humans. Mol Genet Metab 84:160–166
Schiffmann R, van der Knaap MS (2009) Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 72:750–759
van der Knaap MS, Pronk JC, Scheper GC (2006) Vanishing white matter disease. Lancet Neurol 5:413–423
Zara F, Biancheri R, Bruno C, Bordo L, Assereto S, Gazzerro E, Sotgia F, Wang XB, Gianotti S, Stringara S, Pedemonte M, Uziel G, Rossi A, Schenone A, Tortori-Donati P, van der Knaap MS, Lisanti MP, Minetti C (2006) Deficiency of hyccin, a newly identified membrane protein, causes hypomyelination and congenital cataract. Nat Genet 38:1111–1113
Bekiesinska-Figatowska M, Mierzewska H, Kuczynska-Zardzewialy A, Szczepanik E, Obersztyn E (2010) Hypomyelination, hypogonadotropic hypogonadism, hypodontia—First Polish patient. Brain Dev 32(7):574–578
Timmons M, Tsokos M, Asab MA, Seminara SB, Zirzow GC, Kaneski CR, Heiss JD, van der Knaap MS, Vanier MT, Schiffmann R, Wong K (2006) Peripheral and central hypomyelination with hypogonadotropic hypogonadism and hypodontia. Neurology 67:2066–2069
Vazquez-Lopez M, Ruiz-Martin Y, de Castro-Castro P, Garzo-Fernandez C, Martin-del VF, Marquez-de la Plata L (2008) Central hypomyelination, hypogonadotrophic hypogonadism and hypodontia: a new leukodystrophy. Rev Neurol 47:204–208
Wolf NI, Harting I, Boltshauser E, Wiegand G, Koch MJ, Schmitt-Mechelke T, Martin E, Zschocke J, Uhlenberg B, Hoffmann GF, Weber L, Ebinger F, Rating D (2005) Leukoencephalopathy with ataxia, hypodontia, and hypomyelination. Neurology 64:1461–1464
Wolf NI, Harting I, Innes AM, Patzer S, Zeitler P, Schneider A, Wolff A, Baier K, Zschocke J, Ebinger F, Boltshauser E, Rating D (2007) Ataxia, delayed dentition and hypomyelination: a novel leukoencephalopathy. Neuropediatrics 38:64–70
Acknowledgements
We wish to thank all family members for their participation. We would also like to thank all the clinicians who referred patients to us and who were not included in this article: Drs. Marie-Emmanuelle Dilenge, Chantal Poulin, Michael Shevell, Amelie Nadeau, Bruno Maranda, Renée-Myriam Boucher, and Jean Mathieu. We also wish to thank Alexandre Montpetit and Pierre Lepage from McGill University and Genome Quebec Innovation Center for their technical expertise. Dr. Bernard has received a scholarship grant from the Réseau de medicine génique appliquée (RMGA) and from the Fonds de Recherche en Santé du Québec (FRSQ). I. Thiffault has received a scholarship grant from CIHR and ETP fellowship from the National Bank Financial Group. Dr. Vanderver has received funding from the American Academy of Neurology Foundation Clinical Research Training Fellowship program.
Funding information
This research project was supported by the Quebec-base foundation: “Fondation sur les leukodystrophies” (http://www.leucofondation.com/).
Integrity of research and reporting
Ethical standards The experiments comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Table S1
(DOC 177 kb)
Rights and permissions
About this article
Cite this article
Bernard, G., Thiffault, I., Tetreault, M. et al. Tremor–ataxia with central hypomyelination (TACH) leukodystrophy maps to chromosome 10q22.3–10q23.31. Neurogenetics 11, 457–464 (2010). https://doi.org/10.1007/s10048-010-0251-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-010-0251-8