Abstract
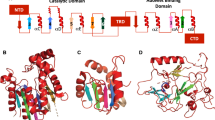
M.HgiDII is a methyltransferase (MTase) from Herpetosiphon giganteus that recognizes the sequence GTCGAC. This enzyme belongs to a group of MTases that share a high degree of amino acid similarity, albeit none of them has been thoroughly characterized. To study the catalytic mechanism of M.HgiDII and its interactions with DNA, we performed molecular dynamics simulations with a homology model of M.HgiDII complexed with DNA and S-adenosyl-methionine. Our results indicate that M.HgiDII may not rely only on Glu119 to activate the cytosine ring, which is an early step in the catalysis of cytosine methylation; apparently, Arg160 and Arg162 may also participate in the activation by interacting with cytosine O2. Another residue from the catalytic site, Val118, also played a relevant role in the catalysis of M.HgiDII. Val118 interacted with the target cytosine and kept water molecules from accessing the region of the catalytic pocket where Cys79 interacts with cytosine, thus preventing water-mediated disruption of interactions in the catalytic site. Specific recognition of DNA was mediated mainly by amino acids of the target recognition domain, although some amino acids (loop 80–88) of the catalytic domain may also contribute to DNA recognition. These interactions involved direct contacts between M.HgiDII and DNA, as well as indirect contacts through water bridges. Additionally, analysis of sequence alignments with closely related MTases helped us to identify a motif in the TRD of M.HgiDII that may be relevant to specific DNA recognition.







Similar content being viewed by others
References
Kozbial PZ, Mushegian AR (2005) Natural history of S-adenosylmethionine-binding proteins. BMC Struct Biol 5:19. doi:10.1186/1472-6807-5-19
Bheemanaik S, Reddy YVR, Rao DN (2006) Structure, function and mechanism of exocyclic DNA methyltransferases. Biochem J 399:177–190
Bujnicki JM (2001) Understanding the evolution of restriction-modification systems: clues from sequence and structure comparisons. Acta Biochim Pol 48:935–967
Tock MR, Dryden DTF (2005) The biology of restriction and anti-restriction. Curr Opin Microbiol 8:466–472
Buryanov Y, Shevchuk T (2005) The use of prokaryotic DNA methyltransferases as experimental and analytical tools in modern biology. Anal Biochem 338:1–11
Klimasauskas S, Weinhold E (2007) A new tool for biotechnology: AdoMet-dependent methyltransferases. Trends Biotechnol 25:99–104
Jeltsch A (2002) Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. ChemBioChem 3:274–293
Malone T, Blumenthal RM, Cheng X (1995) Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol 253:618–632
Estabrook RA, Lipson R, Hopkins B, Reich N (2004) The coupling of tight DNA binding and base flipping: identification of a conserved structural motif in base flipping enzymes. J Biol Chem 279:31419–31428
Klimasauskas S, Kumar S, Roberts RJ, Cheng X (1994) HhaI methyltransferase flips its target base out of the DNA helix. Cell 76:357–369
Huang N, Banavali NK, MacKerell AD (2003) Protein-facilitated base flipping in DNA by cytosine-5-methyltransferase. Proc Natl Acad Sci USA 100:68–73
Estabrook RA, Nguyen TT, Fera N, Reich NO (2009) Coupling Sequence-specific Recognition to DNA Modification. J Biol Chem 284:22690–22696
Zhou H, Purdy MM, Dahlquist FW, Reich NO (2009) The recognition pathway for the DNA cytosine methyltransferase M.HhaI. Biochemistry 48:7807–7816
Estabrook RA, Reich N (2006) Observing an induced-fit mechanism during sequence-specific DNA methylation. J Biol Chem 281:37205–37214
Shieh F, Reich NO (2007) AdoMet-dependent methyl-transfer: Glu119 is essential for DNA C5-cytosine methyltransferase M.HhaI. J Mol Biol 373:1157–1168
Shieh F, Youngblood B, Reich NO (2006) The role of Arg165 towards base flipping, base stabilization and catalysis in M.HhaI. J Mol Biol 362:516–527
Zhang X, Bruice TC (2006) The mechanism of M.HhaI DNA C5 cytosine methyltransferase enzyme: a quantum mechanics/molecular mechanics approach. Proc Natl Acad Sci USA 103:6148–6153
Lau EY, Bruice TC (1999) Active site dynamics of the HhaI methyltransferase: insights from computer simulation. J Mol Biol 293:9–18
Roberts RJ (1994) An amazing distortion in DNA induced by a methyltransferase. Biosci Rep 14:103–117
Szegedi SS, Gumport RI (2000) DNA binding properties in vivo and target recognition domain sequence alignment analyses of wild-type and mutant RsrI [N6-adenine] DNA methyltransferases. Nucleic Acids Res 28:3972–3981
Vilkaitis G, Dong A, Weinhold E, Cheng X, Klimasauskas S (2000) Functional roles of the conserved threonine 250 in the target recognition domain of HhaI DNA methyltransferase. J Biol Chem 275:38722–38730
Neely RK, Roberts RJ (2008) The BsaHI restriction-modification system: cloning, sequencing and analysis of conserved motifs. BMC Mol Biol 9:48. doi:10.1186/1471-2199-9-48
Kröger M, Hobom G, Schütte H, Mayer H (1984) Eight new restriction endonucleases from Herpetosiphon giganteus–divergent evolution in a family of enzymes. Nucleic Acids Res 12:3127–3141
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Bryson K, McGuffin LJ, Marsden RL, Ward LL, Sodhi JS, Jones DT (2005) Protein structure prediction servers at University College London. Nucleic Acids Res 33:W36–38
Eswar N, Eramian D, Webb B, Shen M, Sali A (2008) Protein structure modeling with MODELLER. Methods Mol Biol 426:145–159
Melo F, Devos D, Depiereux E, Feytmans E (1997) ANOLEA: a www server to assess protein structures. Proc Int Conf Intell Syst Mol Biol 5:187–190
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE III, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiórkiewicz-Kuczera J, Yin D, Karplus M (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616
Mackerell A, Banavali N (2000) All-atom empirical force field for nucleic acids: II. Application to molecular dynamics simulations of DNA and RNA in solution. J Comput Chem 21:105–120
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:W407–410
Eisenberg D, Lüthy R, Bowie JU (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
Bourne PE, Weissig H (2003) Structural bioinformatics. Wiley-Liss, Wilmington
Koudan EV, Bujnicki JM, Gromova ES (2004) Homology modeling of the CG-specific DNA methyltransferase SssI and its complexes with DNA and AdoHcy. J Biomol Struct Dyn 22:339–345
Bujnicki JM (2000) Homology modelling of the DNA 5mC methyltransferase M.BssHII. Is permutation of functional subdomains common to all subfamilies of DNA methyltransferases? Int J Biol Macromol 27:195–204
Reinisch K (1995) The crystal structure of HaeIII methyltransferase covalently complexed to DNA: An extrahelical cytosine and rearranged base pairing. Cell 82:143–153
Darii MV, Cherepanova NA, Subach OM, Kirsanova OV, Raskó T, Slaska-Kiss K, Kiss A, Deville-Bonne D, Reboud-Ravaux M, Gromova ES (2009) Mutational analysis of the CG recognizing DNA methyltransferase SssI: insight into enzyme-DNA interactions. Biochim Biophys Acta. doi:10.1016/j.bbapap. 2009.07.016
Gabbara S, Sheluho D, Bhagwat AS (1995) Cytosine methyltransferase from Escherichia coli in which active site cysteine is replaced with serine is partially active. Biochemistry 34:8914–8923
Kumar S, Horton JR, Jones GD, Walker RT, Roberts RJ, Cheng X (1997) DNA containing 4′-thio-2′-deoxycytidine inhibits methylation by HhaI methyltransferase. Nucleic Acids Res 25:2773–2783
Lauster R, Trautner TA, Noyer-Weidner M (1989) Cytosine-specific type II DNA methyltransferases. A conserved enzyme core with variable target-recognizing domains. J Mol Biol 206:305–312
Wintjens R, Liévin J, Rooman M, Buisine E (2000) Contribution of cation-pi interactions to the stability of protein-DNA complexes. J Mol Biol 302:395–410
Reddy CK, Das A, Jayaram B (2001) Do water molecules mediate protein-DNA recognition? J Mol Biol 314:619–632
Rhodes D, Schwabe JW, Chapman L, Fairall L (1996) Towards an understanding of protein-DNA recognition. Philos Trans R Soc Lond B Biol Sci 351:501–509
Acknowledgments
RMR-A is grateful for support to this work from the Instituto Politécnico Nacional (IPN), SIP grant number 20091190, and is recipient of COFAA and EDD fellowships, granted by the IPN. JAC gives thanks to the Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) and the IPN for graduate study fellowships. JAC thanks support from the Comité Técnico de Prestaciones a Becarios (COTEPABE, IPN). During the realization of this work, JAC received financial support from the Research Participation Program at the Center for Biologics Evaluation and Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. The authors are greatly indebted to Juan L. Arciniega (Food and Drug Administration) and the NIH Helix Systems group for providing access to high-performance computational resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castelán-Vega, J.A., Jiménez-Alberto, A. & Ribas-Aparicio, R.M. Homology modeling and molecular dynamics simulations of HgiDII methyltransferase in complex with DNA and S-adenosyl-methionine: Catalytic mechanism and interactions with DNA. J Mol Model 16, 1213–1222 (2010). https://doi.org/10.1007/s00894-009-0632-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0632-9