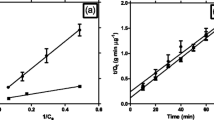
Abstract
Three strains were isolated from hydrocarbon-polluted alpine habitats and were representatives of Cryptococcus terreus (strain PB4) and Rhodotorula creatinivora (strains PB7, PB12). All three strains synthesized and accumulated glycogen (both acid- and alkali-soluble) and trehalose during growth in complex medium containing glucose as carbon source and in minimal salt medium (MSM) with phenol as sole carbon and energy source. C. terreus strain PB4 showed a lower total accumulation level of storage compounds and a lower extracellular polysaccharides (EPS) production than the two R. creatinivora strains, PB7 and PB12. Biofilm formation and phenol degradation by yeast strains attached to solid carriers of zeolite or filter sand were studied at 10°C. Phenol degradation by immobilized yeast strains was always higher on zeolite compared with filter sand under normal osmotic growth conditions. The transfer of cells immobilized on both solid supports to a high osmotic environment decreased phenol degradation activity by all strains. However, both R. creatinivora PB7 and PB12 strains maintained higher ability to degrade phenol compared with C. terreus strain PB4, which almost completely lost its phenol degradation activity. Moreover, R. creatinivora strain PB7 showed the highest ability to form biofilm on both carriers under high osmotic conditions of cultivation.



Similar content being viewed by others
References
Aleksieva Z, Ivanova D, Godjevargova T, Atanasov B (2002) Degradation of some phenol derivates by Trichosporon cutaneum. Process Biochem 37:1215–1219
Allison DG (1993) Biofilm-associated exopolysaccharides. Microbiol Eur 1:16–19
Bastos AE, Moon DH, Rossi A, Trevors JT, Tsai SM (2000) Salt-tolerant phenol-degrading microorganisms isolated from Amazonian soil samples. Arch Microbiol 174:346–352
Bergauer P, Fonteyne PA, Nolard N, Schinner F, Margesin R (2005) Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts. Chemosphere 59:909–918
Chang YH, Li CT, Chang MC (1998) Batch phenol degradation by Candida tropicalis and its fusant Biotechnol Bioeng 60:391–395
Coplin DL, Cook D (1990) Molecular genetics of extracellular polysaccharides biosynthesis in vascular phytopathogenic bacteria. Mol Plant Microbe Interact 3:271–279
Chung TS, Loh KC, Goh SK (1998) Development of cellulose acetate membrane for bacteria immobilization to remove phenol. J Appl Polym Sci 68:1677–1688
Ehrhardt HM, Rehm HJ (1985) Phenol degradation by microorganisms adsorbed on activated carbon. Appl Microbiol Biotechnol 21:32–36
Francois J, Neves MJ, Hers HG (1991) The control of trehalose biosynthesis in Saccharomyces cerevisiae: evidence for a catabolic inactivation and repression of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Yeast 7:575–587
Garcia MJ, Rios G, Ali R, Belles JM, Serrano R (1997) Comparative physiology of salt tolerance in Candida tropicalis and Saccharomyces cerevisiae. Microbiology 143:1125–1131
Gerhardt P (ed) (1981) Manual of methods for general bacteriology. American Society for Microbiology, WA, pp173–174
Grinberg AE, Clesceri LS, Eaton AD (eds) (1992) Phenols. In: Standard methods for the examination of water and wastewater, 18th edn. WA, pp5-30–5-33
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 5B. Academic Press, NY, pp210–344
Hohmann S (1997) Shaping up: the response of yeast to osmotic stress. In: Hohmann S, Mager WH (eds) Yeast stress responses. RG Landes Company, Austin TX, pp 101–145
Hounsa CG, Brandt EV, Thevelein J, Hohmann S, Prior BA (1998) Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 144(Pt 3):671–680
Juares-Ramirez C, Ruiz-Ordaz N, Cristiani-Urbina E, Galindez-Mayer J (2001) Degradation kinetics of phenol by immobilized cells of Candida tropicalis in a fluidised bed reactor. World J Microbiol Biotechnol 17:697–705
Keweloh H, Heipieper HJ, Rehm HJ (1989) Protection of bacteria against toxicity by immobilization in calcium alginate. Appl Microbiol Biotechnol 31:383–389
Kurtz A, Crow SA Jr (1997) Transformation of chlororesorcinol by the hydrocarbonoclastic yeasts Candida maltosa, Candida tropicalis and Trichosporon iovide. Curr Microbiol 35:165–168
Margesin R, Gander S, Zacke G, Gounot AM, Schinner F (2003) Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 7:451–458
Margesin R, Fonteyne PA, Redl B (2005) Low temperature biodegradation of high amounts of phenol by Rhodococcus spp. and basidiomycetous yeasts. Res Microbiol 156:68–75
Meyer AF, Lipson DA, Martin AP, Schadt CW, Schmidt SK (2004) Molecular and metabolic characterization of cold-tolerant alpine soil Pseudomonas sensu stricto. Appl Environ Microbiol 70:483–489
Nichols WW, Evans MJ, Slack MPE, Walmsley HL (1989) The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol 135:1291–1303
Panek A (1963) Function of trehalose in baker’s yeast (Saccharomyces cerevisiae). Arch Biochem Biophys 100:422–425
Pardo C, Lapena MA, Gacto M (1991) On the role of trehalose in yeast cells subjected to hyper osmotic shock. Microbiologia 7:42–48
Parrou JL, Teste MA, Francois J (1997) Effect of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiology 143:1891–1900
Ruiz-Ordaz N, Hernandez-Manzano E, Ruiz-Lagunez JC, Cristiani-Urbina E, Galindez-Mayer J (1998) Growth kinetic model that describes the inhibitory and lytic effects of phenol on Candida tropicalis yeast. Biotechnol Prog 14:966–969
Sampaio JP (1999) Utilization of low molecular weight aromatic compounds by heterobasidiomycetous yeasts: taxonomic implications. Can J Microbiol 45:491–512
Savenkova L, Gercberga Z, Muter O, Nikolaeva V, Dzene A, Tupureina V (2002) PHB-based films as matrices for pesticides. Process Biochem 37:719–722
Stewart PR (1975) Analytical methods for yeasts. In: Prescott DM (ed) Methods of cell biology, vol 12. Academic Press, NY, pp111–147
Sutherland I (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Thevelein JM (1984) Regulation of trehalose mobilization in fungi. Microbiol Rev 48:42–59
Trevelyan WE, Harrison JS (1956) Studies on yeast metabolism 7 Yeast carbohydrate fraction Separation from nucleic acid, analysis and behaviour during anaerobic fermentation. Biochem J 63:23–33
Vandevivere P, Kirchman DL (1993) Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl Environ Microbiol 59:3280–3286
van Dijck P, Colavizza D, Smet P, Thevelein JM (1995) Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl Environ Microbiol 61:109–115
Wase DAJ, Hough JS (1966) Continuous culture yeast on phenol. J Gen Microbiol 42:13–23
Wiemken A (1990) Trehalose in yeast, stress protectants rather than reserve carbohydrate. Antonie Van Leeuwenhoek 58:209–217
Zaicev GM (1995) Utilization of halogenated benzenes, phenols and benzoates by Rhodococcus opacus GM-14. Appl Environ Microbiol 61:4191–4193
Acknowledgement
This work was funded by fifth framework programme in a frame of EC project QLRT-2000-00163 “Multifunctional permeable barriers carrying well-performing microbial biofilms for treatment of mixed pollutant plumes.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Krallish, I., Gonta, S., Savenkova, L. et al. Phenol degradation by immobilized cold-adapted yeast strains of Cryptococcus terreus and Rhodotorula creatinivora . Extremophiles 10, 441–449 (2006). https://doi.org/10.1007/s00792-006-0517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0517-0