Abstract
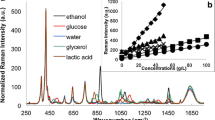
We monitored alcoholic fermentation in Saccharomyces cerevisiae as a function of high hydrostatic pressure. Ethanol production from 0.15 M glucose was measured by Raman spectroscopy in situ in a diamond-anvil cell. At 10 MPa, fermentation proceeds three times faster than at ambient pressure and the fermentation yield is enhanced by 5% after 24 h. Above 20 MPa, the reaction kinetics slows down with increasing pressure. The pressure above which no more ethanol is produced is calculated to be 87 ± 7 MPa. These results indicate that the activity of one or several enzymes of the glycolytic pathway is enhanced at low pressure up to 10 MPa. At higher pressures, they become progressively repressed, and they are completely inhibited above 87 MPa. Although fermentation was predicted to stop at ca. 50 MPa, due to the loss of activity of phosphofructokinase, the present study demonstrates that there is still an activity of ca. 30% of that measured at ambient pressure at 65 MPa. This study also validates the use of Raman spectroscopy for monitoring the metabolism of living microorganisms.





Similar content being viewed by others
References
Aarnoutse PJ, Westerhuis JA (2005) Quantitative Raman reaction monitoring using the solvent as internal standard. Anal Chem 77:1228–1236
Abe F (2004) Piezophysiology of yeast: occurence and significance. Cell Mol Biol 50:437–445
Abe F, Horikoshi K (1995) Hydrostatic pressure promotes the acidification of vacuoles in Saccharomyces cerevisiae. FEMS Microbiol Lett 130:307–312
Abe F, Horikoshi K (1997) Vacuolar acidification in Saccharomyces cerevisiae induced by elevated hydrostatic pressure is transient and is mediated by vacuolar H+-ATPase. Extremophiles 1:89–93
Abe F, Horikoshi K (1998) Analysis of intracellular pH in the yeast Saccharomyces cerevisiae under elevated hydrostatic pressure: a study in baro- (piezo-) physiology. Extremophiles 2:223–228
Bale S, Goodman K, Rochelle P, Marchesi J, Fry J, Weightman A, Parkes R (1997) Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol 47:515–521
Blanco M, Peinado AC, Mas J (2004) Analytical monitoring of alcoholic fermentation using NIR spectroscopy. Biotechnol Bioeng 88:536–542
Daniel I, Oger PM, Picard A, Cardon H, Chervin J-C (2006) A diamond anvil cell adapted for low-pressure high-resolution investigations. Rev Sci Instr (in press)
DeLong E, Yayanos A (1987) Properties of the glucose transport system in some deep-sea bacteria. Appl Environ Microbiol 53:527–532
Fernandes PM (2005) How does yeast respond to pressure? Braz J Med Biol Res 38:1239–1245
Fernandes PM, Domitrovic T, Kao CM, Kurtenbach E (2004) Genomic expression pattern in Saccharomyces cerevisiae cells in response to high hydrostatic pressure. FEBS Lett 556:153–160
Finn B, Harvey LM, McNeil B (2006) Near-infrared spectroscopic monitoring of biomass, glucose, ethanol and protein content in a high cell density baker’s yeast fed-batch bioprocess. Yeast 23:507–517
Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG (1996) Life with 6000 genes. Science 274:546–567
Hofmann E, Kopperschlager G (1982) Phosphofructokinase from yeast. Meth Enzymol 90:49–60
Iwahashi H, Kaul SC, Obuchi K, Komatsu Y (1991) Induction of barotolerance by heat shock treatment in yeast. FEMS Microbiol Lett 64:325–328
Iwahashi H, Shimizu H, Odani M, Komatsu Y (2003) Piezophysiology of genome wide gene expression levels in the yeast Saccharomyces cerevisiae. Extremophiles 7:291–298
Iwahashi H, Odani M, Ishidou E, Kitagawa E (2005) Adaptation of Saccharomyces cerevisiae to high hydrostatic pressure causing growth inhibition. FEBS Lett 579:2847–2852
Kallmeyer J, Boetius A (2004) Effects of temperature and pressure on sulfate reduction and anaerobic oxidation of methane in hydrothermal sediments of guaymas basin. Appl Environ Microbiol 70:1231–1233
Kallmeyer J, Ferdelman TG, Jansen KH, Jorgensen BB (2003) A high-pressure thermal gradient block for investigating microbial activity in multiple deep-sea samples. J Microbiol Meth 55:165–172
Kobori H, Sato M, Tameike A, Hamada K, Shimada S, Osumi M (1995) Ultrastructural effects of pressure stress to the nucleus in Saccharomyces cerevisiae: a study by immunoelectron microscopy using frozen thin sections. FEMS Microbiol Lett 132:253–258
Malone AS, Wick C, Shellhammer TH, Courtney PD (2003) High pressure effects on proteolytic and glycolytic enzymes involved in cheese manufacturing. J Dairy Sci 86:1139–1146
Michels P, Clark DS (1997) Pressure-enhanced activity and stability of a hyperthermophilic protease from a deep-sea methanogen. Appl Environ Microbiol 63:3985–3991
Miller JF, Shah NN, Nelson CM, Ludlow JM, Clark DS (1988) Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschii. Appl Environ Microbiol 54:3039–3042
Mozhaev V, Lange R, Kudryashova E, Balny C (1996) Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol Bioeng 52:320–331
Oger PM, Daniel I, Picard A (2006) Development of a low-pressure diamond anvil cell and analytical tools to monitor microbial activities in situ under controlled P and T. Biochim Biophys Acta 1764:434–442
Parkes RJ, Cragg BA, Bale SJ, Goodman K, Fry JC (1995) A combined ecological and physiological approach to studying sulphate reduction within deep marine sediment layers. J Microbiol Meth 23:235–249
Picard A, Oger PM, Daniel I, Cardon H, Montagnac G, Chervin J-C (2006) A sensitive pressure sensor for diamond anvil cell experiments up to 2 GPa: FluoSpheres®. J Appl Phys 100 (in press)
Shanmuganathan A, Avery SV, Willetts SA, Houghton JE (2004) Copper-induced oxidative stress in Saccharomyces cerevisiae targets enzymes of the glycolytic pathway. FEBS Lett 556:253–259
Acknowledgments
We thank Hervé Cardon and Jean-Claude Chervin for technical assistance in the diamond-anvil cell technology, and the two anonymous reviewers for their helpful comments. This work was supported by grants from the CNRS MRCT and from the CNRS interdisciplinary French program Geomex.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi.
Rights and permissions
About this article
Cite this article
Picard, A., Daniel, I., Montagnac, G. et al. In situ monitoring by quantitative Raman spectroscopy of alcoholic fermentation by Saccharomyces cerevisiae under high pressure. Extremophiles 11, 445–452 (2007). https://doi.org/10.1007/s00792-006-0054-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0054-x