Abstract
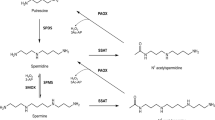
Spermine oxidase (SMO) and acetylpolyamine oxidase (APAO) are FAD-dependent enzymes that are involved in the highly regulated pathways of polyamine biosynthesis and degradation. Polyamine content is strictly related to cell growth, and dysfunctions in polyamine metabolism have been linked with cancer. Specific inhibitors of SMO and APAO would allow analyzing the precise role of these enzymes in polyamine metabolism and related pathologies. However, none of the available polyamine oxidase inhibitors displays the desired characteristics of selective affinity and specificity. In addition, repeated efforts to obtain structural details at the atomic level on these two enzymes have all failed. In the present study, in an effort to better understand structure–function relationships, SMO enzyme–substrate complex has been probed through a combination of molecular modeling, site-directed mutagenesis and biochemical studies. Results obtained indicate that SMO binds spermine in a similar conformation as that observed in the yeast polyamine oxidase FMS1-spermine complex and demonstrate a major role for residues His82 and Lys367 in substrate binding and catalysis. In addition, the SMO enzyme–substrate complex highlights the presence of an active site pocket with highly polar characteristics, which may explain the different substrate specificity of SMO with respect to APAO and provide the basis for the design of specific inhibitors for SMO and APAO.






Similar content being viewed by others
References
Amendola R, Cervelli M, Fratini E, Polticelli F, Sallustio D, Mariottini P (2009) Spermine metabolism and anticancer therapy. Curr Cancer Drug Targets 9:118–130
Babbar N, Murray-Stewart T, Casero RJ (2007) Inflammation and polyamine catabolism: the good, the bad and the ugly. Biochem Soc Trans 35:300–304
Bas D, Rogers D, Jensen J (2008) Very fast prediction and rationalization of pK(a) values for protein-ligand complexes. Proteins Struct Funct Bioinform 73:765–783
Bellelli A, Cavallo S, Nicolini L, Cervelli M, Bianchi M, Mariottini P, Zelli M, Federico R (2004) Mouse spermine oxidase: a model of the catalytic cycle and its inhibition by N, N1-bis(2, 3-butadienyl)-1, 4-butanediamine. Biochem Biophys Res Commun 322:1–8
Bey P, Bolkenius F, Seiler N, Casara P (1985) N-2, 3-Butadienyl-1, 4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem 28:1–2
Bianchi M, Polticelli F, Ascenzi P, Botta M, Federico R, Mariottini P, Cona A (2006) Inhibition of polyamine and spermine oxidases by polyamine analogues. FEBS J 273:1115–1123
Binda C, Coda A, Angelini R, Federico R, Ascenzi P, Mattevi A (1999) A 30-angstrom-long U-shaped catalytic tunnel in the crystal structure of polyamine oxidase. Structure 7:265–276
Binda C, Angelini R, Federico R, Ascenzi P, Mattevi A (2001) Structural bases for inhibitor binding and catalysis in polyamine oxidase. Biochemistry 40:2766–2776
Binda C, Mattevi A, Edmondson D (2002) Structure-function relationships in flavoenzyme-dependent amine oxidations: a comparison of polyamine oxidase and monoamine oxidase. J Biol Chem 277:23973–23976
Brooks B, Bruccoleri R, Olafson B, States D, Swaminathan S, Karplus M (1983) CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comp Chem 4:187–212
Casero RJ, Marton L (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6:373–390
Casero RA, Pegg AE (2009) Polyamine catabolism and disease. Biochem J 421:323–338
Casero RJ, Wang Y, Stewart T, Devereux W, Hacker A, Smith R, Woster P (2003) The role of polyamine catabolism in anti-tumour drug response. Biochem Soc Trans 31:361–365
Cervelli M, Polticelli F, Federico R, Mariottini P (2003) Heterologous expression and characterization of mouse spermine oxidase. J Biol Chem 278:5271–5276
Cervelli M, Bellini A, Bianchi M, Marcocci L, Nocera S, Polticelli F, Federico R, Amendola R, Mariottini P (2004) Mouse spermine oxidase gene splice variants—nuclear subcellular localization of a novel active isoform. Eur J Biochem 271:760–770
Chaturvedi R, Cheng Y, Asim M, Bussière F, Xu H, Gobert A, Hacker A, Casero RJ, Wilson K (2004) Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem 279:40161–40173
Cohen SS (1998) A guide to the polyamines. Oxford University Press, New York
Deleage G, Geourjon C (1993) An interactive graphic program for calculating the secondary structure-content of proteins from circular-dichroism spectrum. Comput Appl Biosci 9:197–199
Gerner E, Meyskens FJ (2004) Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4:781–792
Goodwin A, Jadallah S, Toubaji A, Lecksell K, Hicks J, Kowalski J, Bova G, De Marzo A, Netto G, Casero RJ (2008) Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 68:766–772
Henderson Pozzi M, Gawandi V, Fitzpatrick P (2009) pH dependence of a mammalian polyamine oxidase: insights into substrate specificity and the role of lysine 315. Biochemistry 48:1508–1516
Huang Q, Liu Q, Hao Q (2005) Crystal structures of Fms1 and its complex with spermine reveal substrate specificity. J Mol Biol 348:951–959
Landry J, Sternglanz R (2003) Yeast Fms1 is a FAD-utilizing polyamine oxidase. Biochem Biophys Res Commun 303:771–776
Larkin M, Blackshields G, Brown N, Chenna R, McGettigan P, McWilliam H, Valentin F, Wallace I, Wilm A, Lopez R, Thompson J, Gibson T, Higgins D (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Laskowski R, MacArthur M, Moss D, Thornton J (1993) PROCHECK—a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
MacKerell AJ, Bashford D, Bellott M, Dunbrack RJ, Evanseck J, Field M, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau F, Mattos C, Michnick S, Ngo T, Nguyen D, Prodhom B, Reiher WI, Roux B, Schlenkrich M, Smith J, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem 102:3586–3616
Pegg AE (2009) Mammalian polyamine metabolism and function. IUBMB Life 61:880–894
Persson L (2009) Polyamine homoeostasis. Essays Biochem 46:11–24
Petrey D, Xiang Z, Tang C, Xie L, Gimpelev M, Mitros T, Soto C, Goldsmith-Fischman S, Kernytsky A, Schlessinger A, Koh I, Alexov E, Honig B (2003) Using multiple structure alignments, fast model building, and energetic analysis in fold recognition and homology modeling. Proteins 53(Suppl 6):430–435
Pledgie A, Huang Y, Hacker A, Zhang Z, Woster P, Davidson N, Casero RJ (2005) Spermine oxidase SMO(PAOh1), not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem 280:39843–39851
Polticelli F, Basran J, Faso C, Cona A, Minervini G, Angelini R, Federico R, Scrutton N, Tavladoraki P (2005) Lys300 plays a major role in the catalytic mechanism of maize polyamine oxidase. Biochemistry 44:16108–16120
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schäffer A, Aravind L, Madden T, Shavirin S, Spouge J, Wolf Y, Koonin E, Altschul S (2001) Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res 29:2994–3005
Schipper R, Penning L, Verhofstad A (2000) Involvement of polyamines in apoptosis. Facts and controversies: effectors or protectors? Semin Cancer Biol 10:55–68
Stavropoulos P, Blobel G, Hoelz A (2006) Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol 13:626–632
Thomas T, Thomas T (2001) Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci 58:244–258
Thomas T, Thomas T (2003) Polyamine metabolism and cancer. J Cell Mol Med 7:113–126
Vujcic S, Diegelman P, Bacchi C, Kramer D, Porter C (2002) Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J 367:665–675
Wallace H, Fraser A, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376:1–14
Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA Jr (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 61:5370–5373
Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster P, Casero RJ (2003) Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun 304:605–611
Waterhouse A, Procter J, Martin D, Clamp M, Barton G (2009) Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191
Wu T, Yankovskaya V, McIntire W (2003) Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J Biol Chem 278:20514–20525
Wu T, Ling K, Sayre L, McIntire W (2005) Inhibition of murine N1-acetylated polyamine oxidase by an acetylenic amine and the allenic amine, MDL 72527. Biochem Biophys Res Commun 326:483–490
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinform 9:40
Acknowledgments
The authors wish to thank the University of Roma Tre for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Tavladoraki and M. Cervelli contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tavladoraki, P., Cervelli, M., Antonangeli, F. et al. Probing mammalian spermine oxidase enzyme–substrate complex through molecular modeling, site-directed mutagenesis and biochemical characterization. Amino Acids 40, 1115–1126 (2011). https://doi.org/10.1007/s00726-010-0735-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0735-8