Abstract
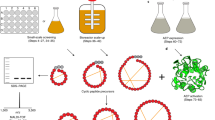
We present the in vivo biosynthesis of wild-type sunflower trypsin inhibitor 1 (SFTI-1) inside E. coli cells using an intramolecular native chemical ligation in combination with a modified protein splicing unit. SFTI-1 is a small backbone cyclized polypeptide with a single disulfide bridge. A small library containing multiple Ala mutants was also biosynthesized and its activity was assayed using a trypsin-binding assay. This study clearly demonstrates the exciting possibility of generating large cyclic peptide libraries in live E. coli cells, and is a critical first step for developing in vivo screening and directed evolution technologies using the cyclic peptide SFTI-1 as a molecular scaffold.




Similar content being viewed by others
Abbreviations
- AMC:
-
7-Amido-4-methyl-coumarin
- CBD:
-
Chitin-binding domain
- Cbz:
-
Benzyloxycarbonyl
- CCK:
-
Cyclic cystine-knot motif
- BBI:
-
Bowman–Birk inhibitor
- EDTA:
-
Ethylenediaminetetraacetic acid
- EtSH:
-
Ethanethiol
- GdmCl:
-
Guanidinium hydrochloride
- GSH:
-
Glutathione
- HPLC:
-
High performance liquid chromatography
- LB:
-
Luria–Bertani
- MAP:
-
Methionyl aminopeptidase
- MESNA:
-
Mercaptoethanesulfonic acid
- NHS:
-
N-hydroxysuccinimide ester
- PAGE:
-
Polyacrylamide gel electrophoresis
- PMSF:
-
Phenylmethylsulfonyl fluoride
- SDS:
-
Sodium dodecyl sulfate
- SFTI-1:
-
Sunflower trypsin inhibitor 1
- TFA:
-
Trifluoroacetic acid
- UV-Vis:
-
Ultraviolet-visible
References
Baird T, Wang B, Lodder M, Hecht S, Craik CS (2000) Generation of active trypsin by chemical cleavage. Tetrahedron 56:9477–9485
Camarero JA, Muir TW (1997) Chemoselective backbone cyclization of unprotected peptides. J Chem Soc Chem Commun 1997:1369–1370
Camarero JA, Muir TW (1999a) Biosynthesis of a head-to-tail cyclized protein with improved biological activity. J Am Chem Soc 121:5597–5598
Camarero JA, Muir TW (1999b) Native chemical ligation of polypeptides. Curr Protoc Protein Sci 18(4):1–21
Camarero JA, Cotton GJ, Adeva A, Muir TW (1998a) Chemical ligation of unprotected peptides directly form a solid support. J Pept Res 51:303–316
Camarero JA, Pavel J, Muir TW (1998b) Chemical synthesis of a circular protein domain: evidence for folding-assisted cyclization. Angew Chem Int Ed 37:347–349
Camarero JA, Fushman D, Cowburn D, Muir TW (2001) Peptide chemical ligation inside living cells: in vivo generation of a circular protein domain. Bioorg Med Chem 9:2479–2484
Camarero JA, Kimura RH, Woo YH, Shekhtman A, Cantor J (2007) Biosynthesis of a fully functional cyclotide inside living bacterial cells. Chembiochem 8:1363–1366
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Craik DJ, Daly NL, Bond T, Waine C (1999) Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol 294:1327–1336
Craik DJ, Simonsen S, Daly NL (2002) The cyclotides: novel macrocyclic peptides as scaffolds in drug design. Curr Opin Drug Discov Dev 5:251–260
Craik DJ, Daly NL, Saska I, Trabi M, Rosengren KJ (2003) Structures of naturally occurring circular proteins from bacteria. J Bacteriol 185:4011–4021
Craik DJ, Cemazar M, Daly NL (2006a) The cyclotides and related macrocyclic peptides as scaffolds in drug design. Curr Opin Drug Discov Dev 9:251–260
Craik DJ, Cemazar M, Wang CK, Daly NL (2006b) The cyclotide family of circular miniproteins: nature’s combinatorial peptide template. Biopolymers 84:250–266
Crovella S, Antcheva N, Zelezetsky I, Boniotto M, Pacor S, Verga Falzacappa MV, Tossi A (2005) Primate beta-defensins—structure, function and evolution. Curr Protein Pept Sci 6:7–21
Daly NL, Chen YK, Foley FM, Bansal PS, Bharathi R, Clark RJ, Sommerhoff CP, Craik DJ (2006) The absolute structural requirement for a proline in the P3′-position of Bowman–Birk protease inhibitors is surmounted in the minimized SFTI-1 scaffold. J Biol Chem 281:23668–23675
Descours A, Moehle K, Renard A, Robinson JA (2002) A new family of beta-hairpin mimetics based on a trypsin inhibitor from sunflower seeds. Chembiochem 3:318–323
Evans TC, Benner J, Xu M-Q (1998) Semisynthesis of cytotoxic proteins using a modified protein splicing element. Protein Sci 7:2256–2264
Evans TC Jr, Martin D, Kolly R, Panne D, Sun L, Ghosh I, Chen L, Benner J, Liu XQ, Xu MQ (2000) Protein trans-splicing and cyclization by a naturally split intein from the dnaE gene of Synechocystis species PCC6803. J Biol Chem 275:9091–9094
Hilpert K, Hansen G, Wessner H, Schneider-Mergener J, Hohne W (2000) Characterizing and optimizing protease/peptide inhibitor interactions, a new application for spot synthesis. J Biochem 128:1051–1057
Hruby VJ, Al-Obeidi F (1990) Emerging approaches in the molecular design of receptor-selective peptide ligands: conformational, topographical and dynamic considerations. J Biochem 268:249–262
Iwai H, Pluckthum A (1999) Circular β-lactamase: stability enhancement by cyclizing the backbone. FEBS Lett 459:166–172
Jaulent AM, Leatherbarrow RJ (2004) Design, synthesis and analysis of novel bicyclic and bifunctional protease inhibitors. Protein Eng Des Sel 17:681–687
Kimura RH, Tran AT, Camarero JA (2006) Biosynthesis of the cyclotide kalata B1 by using protein splicing. Angew Chem Int Ed Engl 45:973–976
Kimura RH, Steenblock ER, Camarero JA (2007) Development of a cell-based fluorescence resonance energy transfer reporter for Bacillus anthracis lethal factor protease. Anal Biochem 369:60–70
Korsinczky ML, Schirra HJ, Rosengren KJ, West J, Condie BA, Otvos L, Anderson MA, Craik DJ (2001) Solution structures by 1H NMR of the novel cyclic trypsin inhibitor SFTI-1 from sunflower seeds and an acyclic permutant. J Mol Biol 311:579–591
Korsinczky ML, Schirra HJ, Craik DJ (2004) Sunflower trypsin inhibitor-1. Curr Protein Pept Sci 5:351–364
Korsinczky ML, Clark RJ, Craik DJ (2005) Disulfide bond mutagenesis and the structure and function of the head-to-tail macrocyclic trypsin inhibitor SFTI-1. Biochemistry 44:1145–1153
Luckett S, Garcia RS, Barker JJ, Konarev AV, Shewry PR, Clarke AR, Brady RL (1999) High-resolution structure of a potent, cyclic proteinase inhibitor from sunflower seeds. J Mol Biol 290:525–533
Marx UC, Korsinczky ML, Schirra HJ, Jones A, Condie B, Otvos L Jr, Craik DJ (2003) Enzymatic cyclization of a potent Bowman–Birk protease inhibitor, sunflower trypsin inhibitor-1, and solution structure of an acyclic precursor peptide. J Biol Chem 278:21782–21789
Muir TW, Sondhi D, Cole PA (1998) Expressed protein ligation: a general method for protein engineering. Proc Natl Acad Sci USA 95:6705–6710
Rizo J, Gierasch LM (1992) Constrained peptides: models of bioactive peptides and protein substructures. Annu Rev Biochem 61:387–418
Scott CP, Abel-Santos E, Wall M, Wahnon D, Benkovic SJ (1999) Production of cyclic peptides and proteins in vivo. Proc Natl Acad Sci USA 96:13638–13643
Severinov K, Muir TW (1998) Expressed protein ligation, a novel method for studying protein–protein interactions in transcription. J Biol Chem 273:16205–16209
Shao Y, Lu WY, Kent SBH (1998) A novel method to synthesize cyclic peptides. Tetrahedron Lett 39:3911–3914
Trabi M, Craik DJ (2002) Circular proteins—no end in sight. Trends Biochem Sci 27:132–138
Wu H, Hu Z, Liu XQ (1998) Protein trans-splicing by a split intein encoded in a split DnaE gene of Synechocystis sp. PCC6803. Proc Natl Acad Sci USA 95:9226–9231
Zhang L, Tam JP (1997) Synthesis and application of unprotected cyclic peptides as building blocks for peptide dendrimers. J Am Chem Soc 119:2363–2370
Acknowledgments
Work was supported by funding from the School of Pharmacy at the University of Southern California and Lawrence Livermore National Laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Austin, J., Kimura, R.H., Woo, YH. et al. In vivo biosynthesis of an Ala-scan library based on the cyclic peptide SFTI-1. Amino Acids 38, 1313–1322 (2010). https://doi.org/10.1007/s00726-009-0338-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0338-4