Abstract
A series of novel 4-chloro-N-(4,5-dihydro-5-oxo-1-R2-1H-1,2,4-triazol-3-yl)-5-methyl-2-(R1-methylthio)benzenesulfonamide derivatives have been synthesized as potential anticancer agents. The in vitro antitumor activity of some compounds was evaluated in the US National Cancer Institute (NCI) against the NCI-60 cell line panel. The most prominent compound showed remarkable activity against 13 human tumor cell lines representing lung, colon, CNS, melanoma, ovarian, renal, prostate, and breast at low micromolar GI50 level in the range of 1.9–3.0 μM.
Graphical Abstract

Similar content being viewed by others
Introduction
The aryl- and heteroarylsulfonamides are widely described compounds revealing a broad spectrum of applications in biological and pharmacological areas [1]. For many years, 2-mercaptobenzenesulfonamide derivatives (MBSAs) have been of interest because of the various biological properties including antitumor [2–10], antimicrobial [11, 12], and antiviral activities [13, 14], and inhibition of carbonic anhydrase [15–17].
It has been known that aryl/heteroarylsulfonamides may act as antitumor agents through a variety of mechanisms such as cell cycle perturbation in the G1 phase, disruption of microtubules, angiogenesis inhibition, and functional suppression of the transcriptional activator NF-Y. The most prominent mechanism was the inhibition of carbonic anhydrase isozymes [18–22]. Recently, a host of structurally novel arylsulfonamide derivatives have been reported to show substantial anticancer activities in vitro and/or in vivo [23–26]. We have reported the synthesis and anticancer activity of 2-mercaptobenzenesulfonamides and subsequently extended our study to analogues with various heterocyclic ring systems attached to the benzenesulfonamide scaffold [4–6, 8, 10, 15] (Fig. 1 structure A [4–6, 8, 15], B [8], C [10]).
In this article we investigated new sulfonamide derivatives containing a triazolone ring in their structure. Triazolones are described in the literature as biologically active compounds, including anti-inflammatories [27], Nki antagonists [28], inhibitors of tumor necrosis factor-α-converting enzyme (TACE) [29], checkpoint kinase-1 inhibitor [30], anti-tumor agents [31–34], and molecular chaperone Hsp90 inhibitor, which is currently in clinical trials for a number of human cancers [35]. Taking into account the interesting properties of triazolones, we have synthesized novel compounds of general structure D (Fig. 1).
Results and discussion
Chemistry
The main goal of this study was to synthesize and investigate the anticancer activity of the new 2-(alkylthio)benzenesulfonamides containing diverse substituted 1,2,4-triazol-5-one moieties. Thus, we propose a synthetic route leading to the target 2-(alkylthio)-4-chloro-N-(4,5-dihydro-5-oxo-1-R2-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamides as shown in Scheme 1.
Starting 3-aminobenzodithiazine 1 could be readily converted to the corresponding dipotassium 2 and potassium salts 3 and 4, according to the reported procedure for preparation of N-(phenylsulfonyl)cyanamide potassium salts [36]. Novel potassium salts 5–10 were prepared by the reaction of 2 with the corresponding halomethyl electrophiles such as aryl/cycloalkyl/methyl chlorides in methanol or ethanol. Subsequent reaction of salts 3–10 with either hydrazine monohydrochloride, methylhydrazine, p-toluenesulfonyl hydrazide, or various 4-substituted phenylhydrazine hydrochlorides led to the formation of the desired 3-(R2-amino)-1-[4-chloro-5-methyl-2-(R1-methylthio)phenylsulfonyl]guanidine derivatives 11–25 as depicted in Scheme 1. It is pertinent to know, however, that aminoguanidine 15 was chosen for the synthesis in two different ways (route A and B in Scheme 1). This was supposed to explain some arising synthetic aspects: whether the usefulness of the potassium salt, i.e., 3 with tosyl hydrazide (route A), is higher than the reaction of aminoguanidine 11 with tosyl chloride (TsCl, route B), and whether the reaction proceeds on the N-terminal nitrogen atom of the sulfonylhydrazide moiety or on the second nitrogen atom neighboring the sulfonyl group. As it turned out, both methods products 15 were identical, with structures (IR, NMR) having a N’-substituted sulfonylhydrazide fragment and obtained in almost equal yields.
Many methods are known for the synthesis of 1,2,4-triazol-5-ones. Triazol-5-ones can be prepared for instance by the reaction of the corresponding nitriles via imidates with semicarbazide [37], from 4-substituted semicarbazides under alkaline conditions [32], by heating of N 1,N 4-substituted hydrazinecarboxamides in alkaline media [38], by cyclization of semicarbazide with an excess of phosgene [39], from the reaction of N-acylureas with arylhydrazines [40], N-acylurethanes with phenylhydrazines as an Einhorn–Brunner reaction extension, as well as from C-halobenzylidenephenylhydrazones via nitrilimines with phenyl isocyanates [41, 42].
In the present study we utilized a new method for the synthesis of 1,2,4-triazol-5-ones in the reaction of the corresponding aminoguanudines 11–25 with an excess of p-toluenesulfonyl isocyanate (TsNCO, Scheme 1). The isocyanates are well known as carbonyl precursors [43] and electrophilic agents whose reactions with hydrazines lead to intramolecular cyclization to five-membered heterocyclic rings [44] or reagents in cycloaddition reactions with various compounds having C=N bonds [45].
Our experiments demonstrated that the proposed synthetic route was an efficient way to prepare the desired N-(4,5-dihydro-5-oxo-1H-1,2,4-triazol-3-yl)benzenesulfonamides 26–40 when an excess of three molar equivalents of tosyl isocyanate was applied in the reaction with the corresponding aminoguanidines 11–25 in anhydrous tetrahydrofuran (THF) for at least 9 h at reflux. It is noteworthy, however, when 2 equivalents of tosyl isocyanate were used, no cyclization product was observed and a complex mixture of products was formed, even after considerable extending of the reaction time.
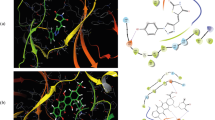
The structure of the new compounds was confirmed by elemental analyses (C, H, N) and spectral (NMR, IR, MS) data presented in the experimental section. Moreover, X-ray analysis was undertaken to confirm proposed structures on the representative compound 31, which crystallized as pyridinium salt (further specified as 31Pyr, Figs. 2 and 3).
Hydrogen bonds in structure of 31Pyr. Blue lines represent hydrogen bonds; transparent yellow balls denote inversion centers in the crystal (generated using Mercury CSD 2.4 [46])
Molecular structure
Details on data collection, structure solution, and refinement are given in Table 1. Compound 31Pyr crystallizes in the monoclinic space group C2/c with (typical for this symmetry) eight molecules in the unit cell. The molecule, being a secondary benzenesulfonamide, is deprotonated at the N1 atom and in the crystal structure is present in the anionic form (Fig. 2). The proton is accepted by pyridine so a pyridinium ion acts as a counterion. Additionally the solid contains solvating molecules of water that reside on twofold rotation axes, and these positions are not fully occupied by them (s.o.f. = 0.079). Actually, only ca. 1/8 of the H2O molecules suffice to fit to the observed electron density in this region.
The two ions are linked by a charge-assisted hydrogen bond of the (+)NH···N(−) type; pyridinium N(5) is a donor, and sulfonamide N(1) is an acceptor. Bonds N(4)–H(4) interact with carbonyl oxygen atoms O5 from the triazolone moiety of the neighboring molecules forming intermolecular hydrogen bonds NH···O. These interactions arranged in pairs can be described by the R 22 (8) motifs situated about local inversion centers (see Fig. 3). Detailed information on hydrogen bonds is given in Table 2. Packing of molecules in the solid state is reinforced also by π–π stacking interactions between adjacent aromatic rings C5–C10 whose centers of gravity (Cg or centroids) are distant at 3.8513(10) Å. The geometry of the interaction is more precisely characterized in Table 3.
Biological assay
Compounds 27, 28, and 30–39 were initially tested at a single dose (10−5 M) in the full NCI-60 cell panel, and the results are shown in Table 4. The methodology of the in vitro cancer screen is described at the website http://www.dtp.nci.nih.gov/branches/btb/ivclsp.html.
The relatively highest sensitivity to the compounds described here was found for the cell lines of non-small cell lung cancer NCI-H522 cell line to compounds 27, 28, 31, and 38 (46 % < IGP < 84 %), leukemia RPMI-8226 to compounds 27, 28, 30, and 31 (36 % < IGP < 45 %), HL-60(TB) to 30, 31, 36, and 39 (22 % < IGP < 91 %), and K-562 to compounds 30, 31, and 38 (37 % < IGP < 66 %) as well as breast MCF7 to 30, 36, and 38 (38 % < IGP < 83 %) (Table 4).
The following conclusions can be drawn from the structure–activity relationship study (Table 4):
-
1.
The susceptibility of the non-small cell lung NCI-H522 cell line against 2-(benzylthio)-N-(2,5-dihydro-5-oxo-1-R2-1H-1,2,4-triazol-3-yl)benzenesulfonamide derivatives (27, 28, 30) was remarkable and increased when the methyl group (R2 = Me, 27, IGP = 46 %) was replaced by aromatic moieties such as phenyl (R2 = Ph, 28, IGP = 69 %) or tosyl (R2 = 4-MePhSO2, 30, IGP = 71 %). The compounds mentioned above showed similar potency for RPMI-8226 (27, IGP = 36 %; 28, IGP = 45 %; 30, IGP = 45 %) and SR (27, IGP = 7 %; 28, IGP = 28 %; 30, IGP = 57 %) lines of leukemia. It should be noted, moreover, that replacement of R2 = Ph (28) for R2 = 4-MePhSO2 (30) caused loss of activity against non-small cell lung cancer (A549/ATCC) and renal (RXF 393) cell lines.
-
2.
For the series of N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-2-(R1-methylthio)benzenesulfonamides the substituent at the sulfur atom S-2 at the 2-position of benzenesulfonamide has an impact on the antiproliferative activity against some cancer cell lines: exchange for instance of R1 = 1-naphthyl in 36 into R1 = Ph (28), 1,3-dioxolan-1-yl (32), 3-CF3Ph (34), 4-CF3Ph (35), and 1,2-dihydro-2-oxoquinolin-4-yl (39) decreased activity against the leukemia HL-60(TB) cell line, as well as the leukemia MOLT-4 cell line; replacing R1 = 1-naphthyl or Ph for R1 = 3-CF3Ph, 4-CF3Ph or 1,2-dihydro-2-oxoquinolin-4-yl resulted in loss of activity against cell lines non-small cell lung A549/ATCC and renal RXF 393.
-
3.
The significant susceptibility of almost the entire colon cancer subpanel against N-(4,5-dihydro-5-oxo-1-R2-1H-1,2,4-triazol-3-yl)-2-(naphthalen-1-ylmethylthio)benzenesulfonamides 36 and 38 should be pointed out. Moreover, the exchange of R2 = Ph (36) or 4-MePhSO2 (38) for 4-ClPh (37) led to a lack of susceptibility of HCC-2998, HTC-116, HTC-15, HT29, and SW-620.
Further anticancer evaluation was performed at five-dose assay on the distinctive compound 36. The anticancer activity of the tested compound was reported for each cell line by the parameters GI50 (molar concentration of the compounds that inhibit 50 % net cell growth), TGI (molar concentration of the compounds leading to total inhibition), and LC50 (molar concentration of the compounds causing 50 % net cell death). The susceptibility of individual subpanels indicates the following order: prostate, colon, CNS, leukemia, ovarian, non-small cell lung, melanoma, renal, and breast cancer (Table 5). As shown in Table 5, compound 36 exhibited remarkable activity at low GI50 level <11.2 μM (MID GI50 = 4.2 μM) over a number of cancer cell lines, acting effectively against 13 human tumor cell lines with GI50 values in the low micromolar range of 1.9–3.0 μM with selectivity toward melanoma MDA-MB-435 (GI50 = 1.9 μM, TGI = 5.5) and renal A498 (GI50 = 1.9 μM, TGI = 10.5) cell lines. It is worth mentioning that lines HL-60(TB), NCI-H522, COLO 205, SF-539, MDA-MB-435, OVCAR-3, A498, RXF 393, DU-145, and MDA-MB-468 were characterized by the relatively low parameters GI50 (1.9–3.2 μM), TGI (4.9–12.3 μM), and LC50 below 58.7 μM.
A COMPARE [47] analysis at the NCI of compound 36 showed a moderate Pearson’s correlation coefficient (PCC = 0.473–0.425) with agents disrupting microtubule formation such as maytansine and rhizoxin [48].
Conclusion
We have developed a new method for the synthesis of a series of 2-(alkylthio)-4-chloro-N-(4,5-dihydro-5-oxo-1-R2-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamides 26–40. The prominent compound 36 showed high (GI50 = 1.9–3.0 μM) activity against 13 of the tumor cell lines and reasonable activity at level GI50 <11.2 μM (MID GI50 = 4.2 μM) over a number cell lines, suggesting that 36 may be a useful lead compound for the search for more powerful anticancer agents with low toxicity against normal cells.
Experimental
The following instruments and parameters were used: melting points: Boetius apparatus; IR spectra: KBr pellets, 400–4,000 cm−1, Thermo Mattson Satellite FTIR spectrometer; 1H NMR and 13C NMR: Varian Gemini 200 apparatus or Varian Unity Plus 500 MHz, chemical shifts are expressed as δ values relative to Me4Si as standard; LC–MS analyses: Shimadzu LCMS-IT-TOF LC-20A mass spectrometer with an electrospray ionization, capillary voltage in positive ion mode +4.5 kV, column: Jupiter 4 u Proteo 90 Å, 4.0 × 150 mm, 4 μm, mobile phase: A—water with 0.1 % formic acid, B—0.1 % formic acid in acetonitrile, linear gradient 50–100 % B in 45 min, flow rate: 0.2 cm3 min−1. The results of elemental analyses for C, H, and N were in agreement with the calculated values within ±0.3 % range. Thin-layer chromatography (TLC) was performed on Merck Kieselgel 60F254 plates and visualized with UV. N-(5-Methylphenylsulfonyl)cyanamide potassium salts 3, 4 and aminoguanidines 11–14 and 16 were obtained in accordance with the previously described procedures [2, 36].
N-[4-Chloro-2-(1,3-dioxolan-2-ylmethylthio)-5-methylphenylsulfonyl]cyanamide potassium salt (5, C12H12ClKN2O4S2)
To a suspension of 3.05 g 5-chloro-2-(cyanoaminosulfonyl)-4-methylthiophenolate dipotassium salt (2, 9 mmol) in 9 cm3 methanol 2.4 cm3 2-(bromomethyl)-1,3-dioxolane (23 mmol) was added dropwise for 5 min. The reaction mixture was stirred at 65 °C for 6.5 h, then 12 h at room temperature. The precipitate was collected by filtration. The filtrate was evaporated to dryness, and the residue was triturated with 90 cm3 diethyl ether to give a second fraction of precipitate. The product was extracted from the combined fractions of solid with hot ethanol to give 2.99 g (86 %) 5. M.p.: 224–225 °C; TLC: R f = 0.74 (CHCl3:MeOH = 3:1); IR (KBr): \( \bar{v} \) = 2,924 (CH3, CH2), 2,854 (CH3, CH2), 2,179 (C≡N), 1,339, 1,145 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.31 (s, 3H, CH3), 3.21 (d, 2H, S–CH2), 3.79–4.00 (m, 4H, CH2–O), 5.11 (t, 1H, CH–O), 7.46 (s, 1H, H-3), 7.77 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.22, 36.04, 64.96, 102.34, 117.50, 127.42, 130.74, 131.36, 135.75, 135.97, 140.86 ppm.
General procedure for the preparation of N-[4-chloro-5-methyl-2-(R1-methylthio)phenylsulfonyl]cyanamide potassium salts 6–10
To a suspension of 3.05 g 5-chloro-2-(cyanoaminosulfonyl)-4-methylthiophenolate dipotassium salt (2, 9 mmol) in methanol or ethanol the appropriate halomethyl electrophile was added. The reaction mixture was stirred at room temperature or at 65 °C. The precipitate was collected by filtration. The product was separated from inorganic salts by extraction with 200 cm3 hot ethanol.
N-[4-Chloro-5-methyl-2-[3-(trifluoromethyl)benzylthio]phenylsulfonyl]cyanamide potassium salt (6, C16H11ClF3KN2O2S2)
Starting from 2 in 45 cm3 ethanol and 1.3 cm3 3-(trifluoromethyl)benzyl chloride (9 mmol) for 2 h at room temperature, compound 6 was obtained. Yield: 3.46 g (84 %); m.p.: 158–160 °C; TLC: R f = 0.87 (CHCl3:MeOH = 2:1); IR (KBr): \( \bar{v} \) = 2,924 (CH3, CH2), 2,174 (C≡N), 1,332, 1,132 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.31 (s, 3H, CH3), 4.41 (s, 2H, S–CH2), 7.41 (s, 1H, H-3), 7.58-7.62 (m, 2H, Ar), 7.76–7.81 (m, 3H, H-6, Ar) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.23, 35.73, 117.46, 124.12, 124.20, 125.95, 126.03, 127.78, 129.77, 130.85, 131.89, 133.49, 134.82, 135.94, 138.57, 141.12 ppm.
N-[4-Chloro-5-methyl-2-[4-(trifluoromethyl)benzylthio]phenylsulfonyl]cyanamide potassium salt (7, C16H11ClF3KN2O2S2)
Starting from 2 in 45 cm3 ethanol and 1.3 cm3 4-(trifluoromethyl)benzyl chloride (9 mmol) for 4 h at room temperature, compound 7 was obtained. Yield: 3.64 g (88 %); m.p.: 177–178 °C; TLC: R f = 0.69 (ethyl acetate:isopropanol = 2:1); IR (KBr): \( \bar{v} \) = 2,921 (CH3, CH2), 2,176 (C≡N), 1,327, 1,137 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.29 (s, 3H, CH3), 4.38 (s, 2H, S–CH2), 7.38 (s, 1H, H-3), 7.66 (d, 2H, Ar), 7.68 (d, 2H, Ar), 7.73 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.23, 35.78, 117.50, 125.41, 125.49, 125.56, 127.57, 130.13, 130.87, 131.84, 134.93, 135.96, 141.06, 142.02 ppm.
N-[4-Chloro-5-methyl-2-(naphthalen-1-ylmethylthio)phenylsulfonyl]cyanamide potassium salt (8, C19H14ClKN2O2S2)
Starting from 2 in 10 cm3 ethanol and 1.3 cm3 1-(chloromethyl)naphthalene (9 mmol) for 1 h at room temperature, compound 8 was obtained. Yield: 3.09 g (78 %); m.p.: 223–225 °C; TLC: R f = 0.63 (ethyl acetate:isopropanol = 2:1); IR (KBr): \( \bar{v} \) = 2,922 (CH3, CH2), 2,175 (C≡N), 1,341, 1,140 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.32 (s, 3H, CH3), 4.72 (s, 2H, S–CH2), 7.44–7.47 (m, 1H, Ar), 7.51 (s, 1H, H-3), 7.52–7.59 (m, 2H, Ar), 7.61 (d, 1H, Ar), 7.76 (s, 1H, H-6), 7.87 (d, 1H, Ar), 7.95 (d, 1H, Ar), 8.24 (d, 1H, Ar) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.29, 34.68, 117.49, 124.62, 125.78, 126.20, 126.52, 127.76, 128.20, 128.36, 128.76, 130.80, 131.60, 131.71, 132.20, 133.68, 136.04, 136.15, 140.76 ppm.
N-[4-Chloro-2-(1,2-dihydro-2-oxoquinolin-4-ylmethylthio)-5-methylphenylsulfonyl]cyanamide potassium salt (9, C18H13ClKN3O3S2)
Starting from 2 in 42 cm3 ethanol and 2.1 g 4-(bromomethyl)quinolin-2(1H)-one (9 mmol) for 4 h at room temperature, compound 9 was obtained. Yield: 3.60 g (88 %); m.p.: 199–201 °C; TLC: R f = 0.61 (ethyl acetate:isopropanol:acetic acid = 1:1:0.02); IR (KBr): \( \bar{v} \) = 2,922 (CH3, CH2), 2,181 (C≡N), 1,668 (CO), 1,341, 1,142 (SO2) m−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.31 (s, 3H, CH3), 4.52 (s, 2H, S-CH2), 6.57 (s, 1H, Ar), 7.21 (t, 1H, Ar), 7.32 (d, 1H, Ar), 7.40 (s, 1H, H-3), 7.51 (t, 1H, Ar), 7.77 (s, 1H, H-6), 7.93 (d, 1H, Ar), 11.74 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.30, 33.33, 115.88, 117.50, 118.47, 121.99, 122.08, 125.42, 128.14, 130.82, 132.21, 134.60, 136.04, 139.22, 141.24, 146.29, 161.59 ppm.
N-[4-Chloro-2-(2,3-dihydrobenzo[b][1,4]dioxin-2-ylmethylthio)-5-methylphenylsulfonyl]cyanamide potassium salt (10, C17H14ClKN2O4S2)
Starting from 2 in 23 cm3 methanol and 1.7 cm3 2-(bromomethyl)-1,4-benzodioxane (12 mmol) for 6 h at 65 °C, compound 10 was obtained. Yield: 3.2 g (78 %); m.p.: 98–100 °C; TLC: R f = 0.86 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 2,923 (CH3, CH2), 2,176 (C≡N), 1,343, 1,143 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.33 (s, 3H, CH3), 3.34 (d, 2H, S–CH2), 4.04–4.13 (m, 2H, CH2–O), 4.29–4.43 (m, 1H, CH–O), 6.80–6.91 (m, 4H, Ar), 7.57 (s, 1H, H-3), 7.78 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.30, 32.96, 66.34, 71.91, 107.38, 117.23, 117.37, 121.61, 121.79, 128.34, 130.85, 132.24, 134.60, 136.20, 141.72, 142.81, 143.13 ppm.
General procedure for the preparation of 1-[4-chloro-5-methyl-2-(R1-methylthio)phenylsulfonyl]-3-(R2-amino)guanidines 15, 17–25
To a suspension of the appropriate N-(phenylsulfonyl)cyanamide potassium salt (3, 5–10, 3.5 mmol) in dry toluene was added the corresponding phenylhydrazine hydrochloride derivative (3.5 mmol) or p-toluenesulfonyl hydrazide (3.5 mmol) in the presence of p-toluenesulfonic acid monohydrate (PTSA, 3.5 mmol). The reaction mixture was stirred at reflux for 1–8 h, and left overnight at 0 °C. The precipitate was filtered off, and dried, then treated with 20 cm3 of water. After vigorously stirring for 30 min the precipitate was collected by filtration, dried, and crystallized from ethanol (15, 17, 19, 21-23, 25), ethyl acetate/hexane (18), or ethyl acetate (20, 24).
1-[2-(Benzylthio)-4-chloro-5-methylphenylsulfonyl]-3-(4-methylphenylsulfonylamino)guanidine (15, C22H23ClN4O4S3)
Method A. According to the general procedure, starting from 1.37 g 3, 0.65 g p-toluenesulfonyl hydrazide, and 0.66 g PTSA in 40 cm3 of dry toluene for 1 h, the title compound 15 was obtained. Yield: 1.62 g (86 %); m.p.: 242–244 °C; TLC: R f = 0.38 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,469, 3,361 (NH), 2,922, 2,832 (CH3, CH2), 1,384, 1,340, 1,172, 1,141 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.30 (s, 3H, CH3), 2.39 (s, 3H, CH3), 4.29 (s, 2H, S–CH2), 7.18 (brs, 1H, NH=), 7.25–7.28 (m, 1H, Ar), 7.32–7.38 (m, 4H, Ar), 7.42–7.43 (m, 4H, H-3, NH, Ar), 7.66 (d, 2H, Ar), 7.79 (s, 1H, H-6), 9.23 (s, 1H, N–NHSO2), 9.89 (s, 1H, SO2NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.20, 21.35, 36.60, 127.51, 128.04, 128.15, 128.74, 129.41, 129.89, 130.58, 132.09, 134.71, 135.86, 136.48, 136.74, 139.64, 144.17, 158.47 ppm.
Method B. To a cooled mixture of 1.35 g 11 (3.5 mmol) in 5 cm3 dry pyridine was added 0.67 g tosyl chloride (3.5 mmol). The ice bath was removed and the mixture was stirred at room temperature for 4 h, then at 60–65 °C for 5 h. After standing overnight, the mixture was added dropwise to 12 cm3 slush and vigorously stirred for 2 h. The solid was filtered off, washed with water (5 × 20 cm3), 1 % HCl (2 × 20 cm3), water (2 × 20 cm3) and dried. Purification from MeOH yielded 15 (86 %); m.p. 242–244 °C (dec.); IR and 1H NMR spectra were identical with an authentic sample of 15.
1-[4-Chloro-2-(1,3-dioxolan-2-ylmethylthio)-5-methylphenylsulfonyl]-3-(phenylamino)guanidine (17, C18H21ClN4O4S2)
Starting from 1.36 g 5 and 0.51 g phenylhydrazine hydrochloride in 5 cm3 dry toluene for 1 h, the title compound 17 was obtained. Yield: 0.80 g (50 %); m.p.: 173–175 °C; TLC: R f = 0.59 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,447 (NH), 2,923 (CH3, CH2), 1,393, 1140 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.32 (s, 3H, CH3), 3.28 (d, 2H, S-CH2), 3.79–3.85 (m, 2H, CH2–O), 3.92–3.98 (m, 2H, CH2–O), 5.14 (t, 1H, CH–O), 6.69 (d, 2H, Ar), 6.79 (t, 1H, Ar), 7.02 (s, 1H, NH=), 7.17 (t, 2H, Ar), 7.38 (s, 1H, NH-Ph), 7.56 (s, 1H, H-3), 7.86 (s, 1H, H-6), 7.88 (s, 1H, NH-NH-Ph), 9.07 (s, 1H, NHSO2) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.22, 36.44, 65.06, 102.35, 112.99, 120.01, 128.16, 129.09, 130.56, 132.04, 136.00, 136.64, 140.28, 148.23, 159.25 ppm.
1-[4-Chloro-5-methyl-2-[3-(trifluoromethyl)benzylthio]phenylsulfonyl]-3-(phenylamino)guanidine (18, C22H20ClF3N4O2S2)
Staring from 1.61 g 6 and 0.50 g phenylhydrazine hydrochloride in 11 cm3 dry toluene for 1 h, the title compound 18 was obtained. Yield: 1.26 g (68 %); m.p.: 184–185 °C; TLC: R f = 0.67 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,444 (NH), 2,925 (CH3, CH2), 1,330, 1,120 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.30 (s, 3H, CH3), 4.46 (s, 2H, S–CH2), 6.66 (d, 2H, Ar), 6.77 (t, 1H, Ar), 7.04 (s, 1H, NH=), 7.13 (t, 2H, Ar), 7.40 (s, 1H, NH-Ph), 7.47 (s, 1H, H-3), 7.55–7.68 (m, 2H, Ar), 7.74 (s, 1H, H-6), 7.97 (s, 1H, NH-NH-Ph), 7.88–7.97 (m, 2H, Ar), 9.08 (s, 1H, NHSO2) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.22, 35.90, 112.94, 119.97, 124.20, 124.27, 126.01, 126.08, 128.42, 129.05, 129.89, 130.77, 132.49, 133.44, 134.90, 136.59, 138.39, 140.47, 148.16, 159.19 ppm.
1-[4-Chloro-5-methyl-2-[3-(trifluoromethyl)benzylthio]phenylsulfonyl]-3-(4-methylphenylsulfonylamino)guanidine (19, C23H22ClF3N4O4S3)
Starting from 1.61 g 6, 0.65 g p-toluenesulfonyl hydrazide, and 0.66 g PTSA in 40 cm3 dry toluene for 1.5 h, the title compound 19 was obtained. Yield: 1.49 g (70 %); m.p.: 190–191 °C; TLC: R f = 0.65 (CHCl3:MeOH = 16:3); IR (KBr): \( \bar{v} \) = 3,459, 3,360, 3,310 (NH), 2,926 (CH3, CH2), 1,635 (C=N), 1,333, 1,174, 1,126 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.29 (s, 3H, CH3), 2.38 (s, 3H, CH3), 4.40 (s, 2H, S–CH2), 7.24 (brs, 1H, NH), 7.36 (d, 2H, Ar tosyl), 7.43 (s, 1H, NH), 7.50–7.77 (m, 7H, Ar, Ar tosyl), 7.78 (s, 1H, H-6), 9.22 (s, 1H, SO2NH), 9.90 (s, 1H, SO2NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.22, 21.32, 36.01, 124.31, 124.39, 126.00, 126.08, 128.13, 128.89, 129.51, 129.89, 130.59, 132.68, 133.46, 134.69, 134.73, 136.69, 138.41, 140.23, 144.18, 158.46 ppm.
1-[4-Chloro-5-methyl-2-[4-(trifluoromethyl)benzylthio]phenylsulfonyl]-3-(phenylamino)guanidine (20, C22H20ClF3N4O2S2)
Starting from 1.61 g 7 and 0.51 g phenylhydrazine hydrochloride in 13 cm3 dry toluene for 2 h, the title compound 20 was obtained. Yield: 1.04 g (56 %); m.p.: 161–164 °C; TLC: R f = 0.71 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,433 (NH), 2,924 (CH3, CH2), 1,325, 1,129 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.29 (s, 3H, CH3), 4.46 (s, 2H, S–CH2), 6.65 (d, 2H, Ar), 6.76 (t, 1H, Ar), 7.04 (s, 1H, NH=), 7.11 (t, 2H, Ar), 7.40 (s, 1H, NH-Ph), 7.46 (s, 1H, H-3), 7.62–7.72 (m, 4H, H-6, Ar, NH-NH-Ph), 7.89 (d, 2H, Ar), 9.06 (s, 1H, NHSO2) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.21, 35.81, 112.94, 119.96, 125.48, 125.56, 125.63, 128.03, 129.04, 130.12, 130.79, 132.39, 135.04, 136.65, 140.29, 141.74, 148.15, 159.21 ppm.
1-[4-Chloro-5-methyl-2-(naphthalen-1-ylmethylthio)phenylsulfonyl]-3-(phenylamino)guanidine (21, C25H23ClN4O2S2)
Starting from 1.54 g 8 and 0.5 g phenylhydrazine hydrochloride in 10 cm3 dry toluene for 1 h, the title compound 21 was obtained. Yield: 0.82 g (40 %); m.p.: 145–150 °C; TLC: R f = 0.71 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,331 (NH), 2,922 (CH3, CH2), 1,391, 1,137 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.33 (s, 3H, CH3), 4.79 (s, 2H, S–CH2), 6.62 (d, 2H, Ar), 6.75 (t, 1H, Ar), 6.96 (s, 1H, NH=), 7.07–7.10 (m, 2H, Ar), 7.33 (s, 1H, NH-Ph), 7.43–7.46 (m, 1H, Ar), 7.52–7.61 (m, 4H, H-3, Ar), 7.84–7.96 (m, 4H, H-6, Ar, NH-NH-Ph), 8.25 (d, 1H, Ar), 9.05 (s, 1H, NHSO2) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.28, 34.82, 112.92, 119.97, 124.50, 125.82, 126.24, 126.60, 128.22, 128.41, 128.80, 128.89, 129.06, 130.73, 131.71, 132.00, 132.24, 133.69, 136.19, 136.72, 140.13, 148.13, 159.19 ppm.
1-[4-Chloro-5-methyl-2-(naphthalen-1-ylmethylthio)phenylsulfonyl]-3-(4-chlorophenylamino)guanidine (22, C25H22Cl2N4O2S2)
Starting from 1.54 g 8 and 0.63 g 4-chlorophenylhydrazine hydrochloride in 10 cm3 dry toluene for 3 h, the title compound 22 was obtained. Yield: 1.34 g (70 %); m.p.: 148–149 °C; TLC: R f = 0.68 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,448, 3,318 (NH), 2,923 (CH3, CH2), 1,340, 1,140 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.33 (s, 3H, CH3), 4.80 (s, 2H, S–CH2), 6.60 (d, 2H, Ar), 6.98 (s, 1H, NH=), 7.07 (d, 2H, Ar), 7.35–7.64 (m, 6H, Ar, NH), 7.84–8.10 (m, 4H, Ar, NH), 8.50 (d, 1H, Ar), 9.05 (s, 1H, NHSO2) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.27, 34.77, 114.35, 123.23, 124.51, 125.81, 126.25, 126.60, 128.27, 128.45, 128.78, 130.72, 131.73, 131.98, 132.22, 133.68, 136.22, 136.77, 139.97, 147.18, 159.01 ppm.
1-[4-Chloro-5-methyl-2-(naphthalen-1-ylmethylthio)phenylsulfonyl]-3-(4-methylphenylsulfonylamino)guanidine (23, C26H25ClN4O4S3)
Starting from 1.54 g 8, 0.65 g p-toluenesulfonyl hydrazide, and 0.66 g PTSA in 70 cm3 dry toluene for 2.5 h, the title compound 23 was obtained. Yield: 1.61 g (78 %); m.p.: 203–206 °C; TLC: R f = 0.32 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,475, 3,370, 3,310 (NH), 2,923 (CH3, CH2), 1633 (C=N), 1,339, 1,172, 1,146 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.33 (s, 3H, CH3), 2.36 (s, 3H, CH3), 4.74 (s, 2H, S–CH2), 7.20 (brs, 1H, NH), 7.31 (d, 2H, Ar tosyl), 7.44 (s, 1H, NH), 7.48–7.74 (m, 6H, Ar naphth, tosyl), 7.66 (s, 1H, H-3), 7.82 (s, 1H, H-6), 7.84–8.02 (m, 2H, Ar naphth), 8.24 (d, 1H, Ar naphth), 9.20 (s, 1H, SO2NH), 9.86 (s, 1H, SO2NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.28, 21.33, 34.97, 124.49, 125.79, 126.24, 126.58, 128.12, 128.22, 128.41, 128.80, 128.96, 129.86, 130.57, 131.67, 132.07, 132.46, 133.68, 134.65, 135.97, 136.79, 139.94, 144.13, 158.45 ppm.
1-[4-Chloro-2-(1,2-dihydro-2-oxoquinolin-4-ylmethylthio)-5-methylphenylsulfonyl]-3-(phenylamino)guanidine (24, C24H22ClN5O3S2)
Starting from 1.6 g 9 and 0.51 g phenylhydrazine hydrochloride in 15 cm3 dry toluene for 8 h, the title compound 24 was obtained. Yield: 1.06 g (58 %); m.p.: 171–173 °C; TLC: R f = 0.70 (CHCl3:MeOH = 16:3); IR (KBr): \( \bar{v} \) = 3,343 (NH), 2,922 (CH3, CH2), 1,663 (CO), 1,386, 1,143 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.31 (s, 3H, CH3), 4.62 (s, 2H, S–CH2), 6.66 (d, 2H, Ar), 6.69 (s, 1H, Ar), 6.76 (t, 1H, Ar), 7.00 (s, 1H, NH =), 7.13 (t, 2H, Ar), 7.22 (t, 1H, Ar), 7.32 (d, 1H, Ar), 7.39 (s, 1H, NH-Ph), 7.50 (t, 1H, Ar), 7.52 (s, 1H, H-3), 7.88 (s, 1H, H-6), 7.90 (s, 1H, NH-NH-Ph), 7.94 (d, 1H, Ar), 9.14 (s, 1H, NHSO2), 11.78 (s, 1H, NH-quinol) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.03, 33.16, 112.69, 115.69, 118.31, 119.75, 121.67, 121.83, 125.01, 128.46, 128.85, 130.51, 130.61, 132.64, 134.39, 136.49, 138.90, 140.48, 146.08, 147.88, 158.91, 161.55 ppm.
1-[4-Chloro-2-(2,3-dihydrobenzo[b][1,4]dioxin-2-ylmethylthio)-5-methylphenylsulfonyl]-3-(phenylamino)guanidine (25, C23H23ClN4O4S2)
Starting from 1.57 g 10 and 0.53 g phenylhydrazine hydrochloride in 8 cm3 dry toluene for 1 h, the title compound 25 was obtained. Yield: 1.12 g (62 %); m.p.: 175–177 °C; TLC: R f = 0.70 (CHCl3:pentane:acetone = 1:1:0.5); IR (KBr): \( \bar{v} \) = 3,442 (NH), 2,923 (CH3, CH2), 1,399, 1,145 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.33 (s, 3H, CH3), 3.32-3.48 (m, 2H, S–CH2), 4.06–4.10 (m, 1H, CH–O), 4.37 (d, 2H, CH2–O), 6.68 (d, 2H, Ar), 6.77 (t, 1H, Ar), 6.81–6.87 (m, 4H, Ar), 7.03 (s, 1H, NH=), 7.15 (t, 2H, Ar), 7.41 (s, 1H, NH-Ph), 7.64 (s, 1H, H-3), 7.88 (s, 1H, H-6), 7.90 (s, 1H, NH-NH-Ph), 9.09 (s, 1H, NHSO2) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.29, 33.19, 66.32, 72.02, 112.96, 117.23, 117.41, 120.03, 121.66, 121.83, 128.87, 129.11, 130.74, 132.78, 134.89, 136.86, 140.95, 142.77, 143.10, 148.19, 159.23 ppm.
General procedure for the preparation of 4-chloro-5-methyl-2-(R1-methylthio)-N-(1-R2-4,5-dihydro-5-oxo-1H-1,2,4-triazol-3-yl)benzenesulfonamide derivatives 26–40
The reaction was carried out in a two-neck round-bottom flask (capacity 5 cm3) with drying tube protection. To the cooled (0 °C) mixture of the corresponding aminoguanidines 11–25 (1 mmol) in dry THF, 0.46 cm3 TsNCO (3 mmol) was added dropwise, and the reaction mixture was stirred at room temperature for 1 h, then at reflux for 8–36 h. After cooling (0 °C, overnight) the reaction product was isolated in precipitate state (27–31, 34–40) or in oil form (26, 32, and 33) and purified by crystallization from ethanol (26-31, 33–39), ethyl acetate (32), or acetonitrile (40).
2-(Benzylthio)-4-chloro-N-(4,5-dihydro-5-oxo-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamide (26, C16H15ClN4O3S2)
Starting from 0.385 g 11 (1 mmol) in 1.5 cm3 THF, the reaction mixture was refluxed for 8 h. After cooling to room temperature, the oily solution was treated with 30 cm3 diethyl ether. The ether solution was decanted from the solid, evaporated to dryness, and the residue crystallized from ethanol to obtain 0.065 g (16 %) of 26. The deposit after decantation was treated with 20 cm3 diethyl ether, filtered off, and purified by crystallization from ethanol to give 0.123 g (30 %) as a second fraction of 26. M.p.: 278–279 °C; TLC: R f = 0.44 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,346 (NH), 2,929 (CH3, CH2), 1,688 (CO), 1,355, 1,161 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.32 (s, 3H, CH3), 4.36 (s, 2H, S–CH2), 7.26 (t, 1H, Ar), 7.32 (t, 2H, Ar), 7.43 (d, 2H, Ar), 7.54 (s, 1H, H-3), 7.86 (s, 1H, H-6), 11.19 (s, 1H, NH), 11.50 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.29, 36.81, 125.89, 127.63, 128.73, 129.49, 132.02, 132.78, 136.05, 136.29, 138.29, 154.50 ppm; LC–MS (IT-TOF): m/z = 410 (M+), t R = 5 min.
2-(Benzylthio)-4-chloro-N-(4,5-dihydro-1-methyl-5-oxo-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamide (27, C17H17ClN4O3S2)
Starting from 0.399 g 12 (1 mmol) in 1.5 cm3 THF, the reaction mixture was refluxed for 8 h. The product was purified to give 0.263 g (62 %) of 27. M.p.: 226–228 °C; TLC: R f = 0.22 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,102 (NH), 2,924 (CH3, CH2), 1,764 (CO), 1,319, 1,131 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.31 (s, 3H, CH3), 3.19 (s, 3H, CH3), 4.32 (s, 2H, S–CH2), 7.25 (t, 1H, Ar), 7.31 (t, 2H, Ar), 7.37 (d, 2H, Ar), 7.51 (s, 1H, H-3), 7.94 (s, 1H, H-6), 11.75 (brs, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.21, 32.66, 36.38, 127.51, 128.04, 128.71, 129.20, 130.93, 132.18, 135.60, 136.67, 136.84, 139.58, 147.70, 152.49 ppm; LC–MS (IT-TOF): m/z = 424 (M+), t R = 6 min.
2-(Benzylthio)-4-chloro-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamide (28, C22H19ClN4O3S2)
Starting from 0.461 g 13 (1 mmol) in 1.5 cm3 THF, the reaction mixture was refluxed for 9 h. The product was purified to give 0.362 g (74 %) of 28. M.p.: 212–214.5 °C; TLC: R f = 0.61 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,240 (NH), 2,923 (CH3, CH2), 1,702 (CO), 1,354, 1,173 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.36 (s, 3H, CH3), 4.34 (s, 2H, S–CH2), 7.12–7.24 (m, 4H, Ar), 7.30–7.44 (m, 4H, Ar), 7.58 (s, 1H, H-3), 7.66 (d, 2H, Ar), 7.98 (s, 1H, H-6), 11.98 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.21, 36.70, 117.66, 124.74, 127.60, 128.49, 128.63, 129.19, 129.44, 132.80, 133.21, 135.55, 135.85, 136.71, 137.91, 138.92, 139.52, 151.52 ppm; LC–MS (IT-TOF): m/z = 486 (M+), t R = 13 min.
2-(Benzylthio)-4-chloro-N-[4,5-dihydro-1-(4-nitrophenylsulfonyl)-5-oxo-1H-1,2,4-triazol-3-yl]-5-methylbenzenesulfonamide (29, C22H18ClN5O7S3)
Starting from 0.596 g 14 (1 mmol) in 2 cm3 THF, the reaction mixture was refluxed for 9 h. The product was purified to give 0.30 g (50 %) of 29. M.p.: 211–214 °C; TLC: R f = 0.59 (benzene:ethanol = 2:1); IR (KBr): \( \bar{v} \) = 3,429, 3,269 (NH), 1,764 (CO), 1,536, 1,350 (NO2), 1,403, 1,391, 1,184, 1,167 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.32 (s, 3H, CH3), 4.28 (s, 2H, S–CH2), 7.10–7.21 (m, 3H, Ar), 7.30 (d, 2H, Ar), 7.50 (s, 1H, H-3), 7.78 (d, 2H, J = 8.8 Hz, Ar), 7.89 (s, 1H, H-6), 8.24 (d, 2H, J = 8.8 Hz, Ar) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.19, 36.41, 124.92, 127.42, 128.30, 128.40, 128.47, 128.82, 129.16, 132.48, 133.47, 135.98, 136.15, 138.37, 141.76, 144.95, 150.91, 151.71 ppm; LC–MS (IT-TOF): m/z = 596 (M+), t R = 15 min.
2-(Benzylthio)-4-chloro-N-[4,5-dihydro-1-(4-methylphenylsulfonyl)-5-oxo-1H-1,2,4-triazol-3-yl]-5-methylbenzenesulfonamide (30, C23H21ClN4O5S3)
Starting from 0.539 g 15 (1 mmol) in 1.5 cm3 THF, the reaction mixture was refluxed for 8 h. The product was purified to give 0.405 g (72 %) of 30. M.p.: 202–204 °C; TLC: R f = 0.60 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,371 (NH), 2,922 (CH3, CH2), 1,755 (CO), 1,387, 1,191, 1,176 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.34 (s, 3H, CH3), 2.37 (s, 3H, CH3), 4.28 (s, 2H, S–CH2), 7.20 (t, 1H, Ar), 7.26 (d, 2H, Ar), 7.33 (d, 2H, Ar), 7.36 (d, 2H, Ar), 7.51 (d, 2H, Ar), 7.54 (s, 1H, H-3), 7.89 (s, 1H, H-6), 11.93 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.23, 21.46, 36.67, 127.32, 127.60, 128.52, 128.66, 129.32, 130.18, 132.69, 133.33, 133.95, 135.42, 135.86, 136.42, 138.77, 143.34, 145.82, 151.67 ppm; LC–MS (IT-TOF): m/z = 564 (M+), t R = 12 min.
4-Chloro-N-[4,5-dihydro-1-(4-methylphenyl)-5-oxo-1H-1,2,4-triazol-3-yl]-2-(ethoxycarbonylmethylthio)-5-methylbenzenesulfonamide (31, C20H21ClN4O5S2)
Starting from 0.471 g 16 (1 mmol) in 1.5 cm3 THF, the reaction mixture was refluxed for 8 h. The precipitate of by-products was filtered off. The filtrate was evaporated to dryness under reduced pressure and purified to give 0.343 g (69 %) of 31. M.p.: 190–191 °C; TLC: R f = 0.42 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,255 (NH), 2,978, 2,801 (CH3, CH2), 1,726 (CO), 1,336, 1,171 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 1.04 (t, 3H, CH3), 2.26 (s, 3H, CH3), 2.38 (s, 3H, CH3), 3.97-4.08 (m, 4H, S–CH2, CH2), 7.18 (d, 2H, Ar), 7.52 (d, 2H, Ar), 7.60 (s, 1H, H-3), 8.01 (s, 1H, H-6), 11.98 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 14.10, 19.23, 20.66, 35.15, 61.43, 117.76, 129.14, 129.54, 133.20, 133.51, 133.90, 135.33, 135.51, 136.11, 138.90, 139.23, 151.40, 168.89 ppm; LC–MS (IT-TOF): m/z = 496 (M+), t R = 12 min.
4-Chloro-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-2-(1,3-dioxolan-2-ylmethylthio)-5-methylbenzenesulfonamide (32, C19H19ClN4O5S2)
Starting from 0.458 g 17 (1 mmol) in 3 cm3 THF, the reaction mixture was refluxed for 5 h. After cooling to room temperature, the oily residue was treated with diethyl ether to obtain a white solid. The crude product was purified to give 0.159 g (33 %) of 32. M.p.: 214–217 °C; TLC: R f = 0.36 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,414 (NH), 2,972 (CH3, CH2), 1,716 (CO), 1,382, 1,165 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.37 (s, 3H, CH3), 3.28 (d, 2H, S–CH2), 3.71-3.75 (m, 2H, CH2–O), 3.83–3.88 (m, 2H, CH2–O), 5.05 (t, 1H, CH–O), 7.13 (t, 1H, Ar), 7.36 (t, 2H, Ar), 7.64 (d, 2H, Ar), 7.68 (s, 1H, H-3), 7.97 (s, 1H, H-6), 11.97 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.22, 36.70, 64.96, 102.10, 117.69, 124.73, 129.18, 129.31, 129.83, 132.98, 136.13, 136.74, 137.89, 138.87, 139.64, 151.54 ppm; LC–MS (IT-TOF): m/z = 482 (M+), t R = 9 min.
4-Chloro-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-5-methyl-2-[3-(trifluoromethyl)benzylthio]benzenesulfonamide (33, C23H18ClF3N4O3S2)
Starting from 0.506 g 18 (1 mmol) in 1 cm3 THF, the reaction mixture was refluxed for 9 h. After cooling to room temperature, the oily residue was treated with diethyl ether to obtain a white solid. The crude product was purified to give 0.210 g (38 %) of 33. M.p.: 195–198 °C; TLC: R f = 0.45 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,425 (NH), 2,924 (CH3, CH2), 1,702 (CO), 1,334, 1,170 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.36 (s, 3H, CH3), 4.48 (s, 2H, S-CH2), 7.14 (t, 1H, Ar), 7.27–7.58 (m, 4H, Ar), 7.58 (s, 1H, H-3), 7.62–7.76 (m, 4H, Ar), 7.98 (s, 1H, H-6), 12.00 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.20, 36.05, 117.62, 124.20, 124.29, 124.71, 126.08, 126.16, 128.93, 129.14, 129.32, 129.56, 133.24, 133.33, 133.43, 135.44, 136.33, 137.85, 138.81, 139.48, 151.49 ppm; LC–MS (IT-TOF): m/z = 554 (M+), t R = 17 min.
4-Chloro-N-[4,5-dihydro-1-(4-methylphenylsulfonyl)-5-oxo-1H-1,2,4-triazol-3-yl]-5-methyl-2-[3-(trifluoromethyl)benzylthio]benzenesulfonamide (34, C24H20ClF3N4O5S3)
Starting from 0.607 g 19 (1 mmol) in 2 cm3 THF, the reaction mixture was refluxed for 36 h. The product was purified to give 0.443 g (71 %) of 34. M.p.: 99–100 °C; TLC: R f = 0.62 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,284 (NH), 2,924 (CH3, CH2), 1,716 (CO), 1,331, 1,347, 1,170, 1,194 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.32 (s, 3H, CH3), 2.34 (s, 3H, CH3), 4.38 (s, 2H, S–CH2), 7.30 (d, 2H, Ar), 7.43–7.52 (m, 4H, Ar), 7.55–7.69 (m, 3H, Ar), 7.86 (s, 1H, H-6), 11.90 (brs, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.24, 21.36, 36.03, 127.34, 127.79, 129.03, 129.78, 129.85, 130.14, 133.12, 133.29, 133.98, 135.36, 136.18, 136.55, 137.83, 138.61, 143.63, 144.62, 145.77, 148.09, 151.75 ppm; LC–MS (IT-TOF): m/z = 632 (M+), t R = 20 min.
4-Chloro-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-5-methyl-2-[4-(trifluoromethyl)benzylthio]benzenesulfonamide (35, C23H18ClF3N4O3S2)
Starting from 0.529 g 20 (1 mmol) in 1 cm3 THF, the reaction mixture was refluxed for 9 h. The product was purified to give 0.29 g (52 %) of 35. M.p.: 208–210 °C; TLC: R f = 0.40 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,253 (NH), 2,923 (CH3, CH2), 1,701 (CO), 1,327, 1,127 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.35 (s, 3H, CH3), 4.46 (s, 2H, S–CH2), 7.14 (t, 1H, Ar), 7.36 (t, 2H, Ar), 7.50 (d, 2H, Ar), 7.56 (d, 2H, Ar), 7.59 (s, 1H, H-3), 7.63 (d, 2H, Ar), 7.97 (s, 1H, H-6), 11.99 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.21, 35.92, 117.62, 124.70, 125.36, 125.43, 127.76, 129.15, 129.83, 130.11, 133.17, 133.25, 135.78, 135.97, 137.88, 138.89, 139.63, 144.53, 151.51 ppm; LC–MS (IT-TOF): m/z = 554 (M+), t R = 17 min.
4-Chloro-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-5-methyl-2-(naphthalen-1-ylmethylthio)benzenesulfonamide (36, C26H21ClN4O3S2)
Starting from 0.513 g 21 (1 mmol) in 1 cm3 THF, the reaction mixture was refluxed for 9 h. After cooling to room temperature, the reaction mixture was treated with petroleum ether to obtain a white solid. The crude product was purified to give 0.166 g (31 %) of 36. M.p.: 214–216 °C; TLC: R f = 0.55 (CHCl3:MeOH = 16:3); IR (KBr): \( \bar{v} \) = 3,258 (NH), 2,922 (CH3, CH2), 1,720 (CO), 1,349, 1,168 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.39 (s, 3H, CH3), 4.80 (s, 2H, S–CH2), 7.15 (t, 1H, Ar), 7.32–7.42 (m, 3H, Ar), 7.51–7.53 (m, 3H, Ar), 7.62–7.73 (m, 3H, H-3, Ar), 7.83 (d, 1H, Ar), 7.92 (d, 1H, Ar), 8.00 (s, 1H, H-6), 8.20 (d, 1H, Ar), 11.90 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.26, 35.02, 117.70, 124.41, 124.78, 125.69, 126.25, 126.62, 128.44, 128.58, 128.78, 129.21, 129.56, 131.43, 131.61, 132.95, 133.11, 133.64, 135.66, 137.08, 137.91, 138.95, 139.51, 151.50 ppm; LC–MS (IT-TOF): m/z = 536 (M+), t R = 18 min.
4-Chloro-N-[1-(4-chlorophenyl)-4,5-dihydro-5-oxo-1H-1,2,4-triazol-3-yl]-5-methyl-2-(naphthalen-1-ylmethylthio)benzenesulfonamide (37, C26H20Cl2N4O3S2)
Starting from 0.545 g 22 (1 mmol) in 2 cm3 THF, the reaction mixture was refluxed for 9 h. The product was purified to give 0.224 g (39 %) of 37. M.p.: 205–206 °C; TLC: R f = 0.59 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,251 (NH), 2,924 (CH3, CH2), 1,722 (C=O), 1,352, 1,166 (SO2) cm−1; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.39 (s, 3H, CH3), 4.81 (s, 2H, S-CH2), 7.31–7.58 (m, 6H, Ar), 7.62–7.72 (m, 3H, Ar), 7.80–7.94 (m, 2H, Ar), 8.01 (s, 1H, H-6), 8.20 (d, 1H, Ar), 11.99 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.27, 34.99, 119.09, 124.41, 125.89, 126.24, 126.61, 128.41, 128.56, 128.77, 129.17, 129.56, 131.43, 131.60, 132.96, 133.09, 133.64, 135.59, 136.76, 137.08, 138.98, 139.92, 142.10, 151.42 ppm; LC–MS (IT-TOF): m/z = 570 (M+), t R = 22 min.
4-Chloro-N-[4,5-dihydro-1-(4-methylphenylsulfonyl)-5-oxo-1H-1,2,4-triazol-3-yl]-5-methyl-2-(naphthalen-1-ylmethylthio)benzenesulfonamide (38, C27H23ClN4O5S3)
Starting from 0.589 g 23 (1 mmol) in 2 cm3 THF, the reaction mixture was refluxed for 11 h. The product was purified to give 0.438 g (70 %) of 38. M.p.: 118–120 °C; TLC: R f = 0.60 (benzene:EtOH = 2:1); IR (KBr): \( \bar{v} \) = 3,530 (NH), 2,973 (CH3, CH2), 1,726 (CO), 1,388, 1,370, 1,193, 1,177 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.31 (s, 3H, CH3), 2.37 (s, 3H, CH3), 4.70 (s, 2H, S–CH2), 7.32 (d, 2H, Ar), 7.38 (t, 1H, Ar), 7.48–7.53 (m, 5H, Ar), 7.63 (s, 1H, H-3), 7.84 (d, 1H, Ar), 7.90 (s, 1H, H-6), 7.94 (d, 1H, Ar), 8.08 (d, 1H, Ar), 11.90 (brs, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.29, 21.42, 35.06, 124.28, 125.76, 126.27, 126.64, 127.37, 128.27, 128.56, 128.79, 128.96, 130.19, 131.38, 131.59, 132.90, 133.27, 133.64, 133.91, 135.68, 136.75, 138.77, 143.56, 145.85, 151.78 ppm; LC–MS (IT-TOF): m/z = 614 (M+), t R = 23 min.
4-Chloro-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-2-(1,2-dihydro-2-oxoquinolin-4-ylmethylthio)-5-methylbenzenesulfonamide (39, C25H20ClN5O4S2)
Starting from 0.528 g 24 (1 mmol) in 3 cm3 THF, the reaction mixture was refluxed for 9 h. The product was purified to give 0.161 g (29 %) of 39. M.p.: 185–188 °C; TLC: R f = 0.12 (CHCl3:MeOH = 16:3), R f = 0.19 (CHCl3:MeCN:AcOH = 2:1:0.05); IR (KBr): \( \bar{v} \) = 3,467 (NH), 2,923 (CH3, CH2), 1,692, 1,655 (CO), 1,383, 1,127 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.37 (s, 3H, CH3), 4.59 (s, 2H, S–CH2), 6.58 (s, 1H, Ar), 7.10 (t, 1H, Ar), 7.16 (t, 1H, Ar), 7.30 (d, 1H, Ar), 7.34 (t, 2H, Ar), 7.49 (t, 1H, Ar), 7.55 (s, 1H, H-3), 7.65 (d, 2H, Ar), 7.88 (d, 1H, Ar), 8.01 (s, 1H, H-6), 11.73 (s, 2H, NH-quinolin, NH-triazolone) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.27, 33.74, 115.88, 117.75, 118.29, 121.90, 122.48, 124.75, 125.28, 129.17, 129.46, 130.83, 133.17, 133.53, 135.51, 136.33, 137.84, 138.90, 139.23, 139.47, 145.59, 151.51, 161.49 ppm; LC–MS (IT-TOF): m/z = 554 (M+), t R = 17 min.
4-Chloro-2-(2,3-dihydrobenzo[b][1,4]dioxin-2-ylmethylthio)-N-(4,5-dihydro-5-oxo-1-phenyl-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamide (40, C24H21ClN4O5S2)
Starting from 0.519 g 25 (1 mmol) in 3 cm3 THF, the reaction mixture was refluxed for 9 h. The product was purified to give 0.262 g (48 %) of 40. M.p.: 185–188 °C; TLC: R f = 0.53 (benzene:ethanol = 2:1); IR (KBr): \( \bar{v} \) = 3,311 (NH), 2,922 (CH3, CH2), 1,697 (CO), 1,334, 1,165 (SO2) cm−1; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.38 (s, 3H, CH3), 3.36 (dd, 1H, S–CH2), 3.46 (dd, 1H, S–CH2), 4.00 (dd, 1H, O–CH2), 4.26 (dd, 1H, O–CH2), 4.31–4.35 (m, 1H, O–CH), 6.70–6.81 (m, 4H, Ar), 7.12 (t, 1H, Ar), 7.34 (t, 2H, Ar), 7.64 (d, 2H, Ar), 7.76 (s, 1H, H-3), 8.01 (s, 1H, H-6), 12.01 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d 6 ): δ = 19.29, 33.64, 66.26, 72.02, 117.15, 117.29, 117.69, 121.61, 121.70, 124.74, 129.14, 129.83, 133.09, 133.61, 135.83, 136.62, 137.83, 139.12, 139.50, 142.61, 142.99, 151.54 ppm; LC–MS (IT-TOF): m/z = 545 (M+), t R = 21 min.
X-ray structure determination
Experimental diffraction data were collected on a KM4 CCD kappa-geometry diffractometer (Oxford diffraction), equipped with a Sapphire2 CCD detector. An enhanced X-ray Mo Kα radiation source with a graphite monochromator was used. Determination of the unit cell and diffraction data collection were carried out at 120 K in a stream of dry nitrogen (Oxford CryoSystems). All calculations (data reduction, structure solution, and refinement) were carried out using CrysAlisPro [49] package. The structure was solved by direct methods, and all non-hydrogen atoms were refined with anisotropic thermal parameters by full-matrix least squares procedure based on F 2. Final refinements were carried out using the SHELX-97 package [50], run under control of WinGX program [51].
All hydrogen atoms were refined using isotropic model with U iso (H) values fixed to be 1.2 times U eq of C atoms for CH and CH2 and 1.5 times U eq for CH3. Bond lengths C–H were fixed at 0.98 Å for methyl groups, and 0.95 Å for methylene and methine groups; distances N–H were set to 0.88 Å. Solvating water molecules generated an electron density peak of ca. 1.7 e Å−3. Because the electron density maximum is placed at a special position (½, y, ¼) localization of hydrogen atoms is additionally uncertain so we did not attempt to find H atoms. The occupation factor of oxygen atom O10 was refined freely to obtain 0.079. One incorrect reflection (−1 1 17) was omitted.
Crystallographic data for the structure of 31Pyr reported in this article have been deposited with the Cambridge Crystallographic Data Center as supplementary publication no. CCDC868805. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [Fax: (+44) 1223-336-033; email: deposit@ccdc.cam.ac.uk].
References
Negwer M (1994) Organic-chemical drugs and their synonyms. Akademie Verlag, Berlin
Sławiński J, Bednarski P, Grünert R, Reszka P (2003) Polish J Chem 77:53
Sławiński J, Bednarski P, Reszka P (2004) Polish J Chem 78:369
Sławiński J (2004) Eur J Med Chem 39:179
Sławiński J, Gdaniec M (2005) Eur J Med Chem 40:377
Sławiński J, Brzozowski Z (2006) Eur J Med Chem 41:1180
Brzozowski Z, Sławiński J (2007) Polish J Chem 81:1419
Brzozowski Z, Sączewski F, Sławiński J (2007) Eur J Med Chem 42:1218
Brzozowski Z, Sączewski F, Sławiński J, Bednarski PJ, Grünert R, Gdaniec M (2007) Bioorg Med Chem 15:2560
Sławiński J, Brożewicz K, Fruziński A, Główka ML (2011) Heterocycles 83:1093
Brzozowski Z, Sławiński J, Kędzia A, Kwapisz E (2009) J Heterocycl Chem 46:1396
Sławiński J, Żołnowska B, Pirska D, Kędzia A, Kwapisz E (2011) J Enzyme Inhib Med Chem. doi:10.3109/14756366.2011.625024
Kuo CL, Assefa H, Brzozowski Z, Sławiński J, Sączewski F, Buolamwini IK, Neamati N (2004) J Med Chem 47:385
Brzozowski Z, Sławiński J, Sączewski F, Sanchez T, Neamati N (2008) Eur J Med Chem 43:1188
Sączewski F, Sławiński J, Kornicka A, Brzozowski Z, Pomarnacka E, Innocenti A, Scozzafava A, Supuran CT (2006) Bioorg Med Chem Lett 16:4846
Sączewski F, Innocenti A, Brzozowski Z, Sławiński J, Pomarnacka E, Kornicka A, Scozzafava A, Supuran CT (2006) J Enzyme Inhib Med Chem 21:563
Sączewski F, Innocenti A, Sławiński J, Kornicka A, Brzozowski Z, Pomarnacka E, Scozzafava A, Supuran CT (2008) Bioorg Med Chem 16:3933
Casini A, Scozzafava A, Mastrolorenzo A, Supuran CT (2002) Curr Cancer Drug Targets 2:55
Supuran CT, Scozzafava A, Casini A (2003) Med Res Rev 23:146
Kivela AJ, Kivela J, Saarnio J, Parkkila J (2005) World J Gastroenterol 11:155
Supuran CT, Scozzafava A (2007) Bioorg Med Chem 15:4336
Supuran CT (2008) Nat Rev Drug Discov 7:168
Supuran CT, Casini A, Mastrolorenzo A, Scozzafava A (2004) Mini Rev Med Chem 4:625
Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT (2004) Bioorg Med Chem Lett 14:217
Rostom SA (2006) Bioorg Med Chem 14:6475
Chen J, Liu T, Wu R, Lou J, Cao J, Dong X, Yang B, He Q, Hu Y (2010) Bioorg Med Chem 18:8478
Mullican MD, Wilson MW, Connor DT, Kostlan CR, Schrier DJ, Dyer RD (1993) J Med Chem 36:1090
Elipe MV, Huskey SE, Zhu B (2003) J Pharm Biomed Anal 30:1431
Sheppeck JE, Gilmore JL, Tebben A, Xue CB, Liu RQ, Decicco CP, Duan JJ (2007) Bioorg Med Chem Lett 17:2769
Oza V, Ashwell S, Brassil P, Breed J, Ezhuthachan J, Deng C, Grondine M, Horn C, Liu D, Lyne P, Newcombe N, Pass M, Read J, Su M, Toader D, Yu D, Yu Y, Zabludoff S (2012) Bioorg Med Chem Lett 22:2330
Demirbaş N, Ugurluoglu R, Demirbaş A (2002) Bioorg Med Chem 10:3717
Pomarnacka E, Koźlarska-Kędra I (2003) Il Farmaco 58:423
Adams ND, Aquino CJ, Chaudhari AM, Ghergurovich JM, Kiesow TJ, Parrish CA, Reif AJ, Wiggall K (2011) Triazolones as fatty acid synthase inhibitors. WO patent 2011103546, 25 Aug 2011
Adams ND, Aquino CJ, Chaudhari AM, Ghergurovich JM, Kiesow TJ, Parrish CA, Reif AJ, Wiggall K (2011) Chem Abstr 155:380343
Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, Zhou D, Inoue T, Tatsuta N, Sang J, Ye S, Acquaviva J, Ogawa LS, Wada Y, Barsoum J, Koya K (2012) Mol Cancer Ther 11:475
Sławiński J (2001) Pol J Chem 75:1309
Sheppeck JE, Gilmore JL, Tebben A, Xue CHB, Liu RQ, Decicco CP, Duan JJW (2007) Bioorg Med Chem Lett 17:2769
Shalini M, Yogeeswari P, Sriram D, Stables JP (2009) Biomed Pharmacother 63:187
Brown RJ, Annis G, Casalnuovo A, Chan D, Shapiro R, Marshall WJ (2004) Tetrahedron 60:4361
Brenner M, Bechtel WD, Palluk R, Wienrich M, Weiser NO (2002) Pharmaceutical compositions containing triazolones and methods of treating neurodegenerative disease using triazolones. US patent 6,492,407 B2, 10 Dec 2002
Huisgen R (1961) Proc Chem Soc 357
Galishev VA, Chistoklevtov UN, Petrov AA (1980) Russ Chem Res 49:880
Kuninobu Y, Nishimura S, Takai K (2006) Org Biomol Chem 4:203
Tsuge O, Kanemasa S, Tashiro M (1968) Tetrahedron 24:5205
Tsuge O, Kanemasa S (1972) Bull Chem Soc Jpn 45:2877
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Crystallogr 41:466
Boyd RM, Paull KD (1995) Drug Dev Res 34:91
Amos LA (2011) Semin Cell Dev Biol 22:916
Oxford Diffraction (2005) CrysAlis CCD and CrysAlis RED, Version 1.171. Oxford Diffraction Ltd, Abingdon, England
Sheldrick GM (2008) Acta Crystallogr A 64:112
Farrugia LJ (1999) J Appl Crystallogr 32:837
Acknowledgments
The authors are very grateful to Dr. Joel Morris, Ph.D., Chief Drug Synthesis & Chemistry Branch, National Cancer Institute (Bethesda, MD), for the in vitro anticancer screening.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sławiński, J., Żołnowska, B., Orlewska, C. et al. Synthesis and molecular structure of novel 2-(alkylthio)-4-chloro-N-(4,5-dihydro-5-oxo-1H-1,2,4-triazol-3-yl)-5-methylbenzenesulfonamides with potential anticancer activity. Monatsh Chem 143, 1705–1718 (2012). https://doi.org/10.1007/s00706-012-0849-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0849-7