Abstract
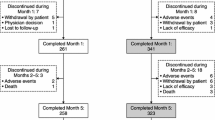
To compare the efficacy and safety of levodopa/carbidopa/entacapone (LCE) with levodopa/carbidopa (LC) on Parkinson’s disease (PD) patients with mild, or only minimally disabling motor complications. A prospective 3-month, multicentre, parallel-group, double-blind, and randomised phase IV study was performed. The primary endpoint was to assess the efficacy of LCE compared to LC on ADLs using the UPDRS part II. Secondary endpoints were assessed by the UPDRS (I, III and IV) scores, QUICK and PDQ-39 questionnaires, and patient and investigator clinical global impression (CGI). Ninety-five patients were randomly assigned to treatment with LCE (100/25/200 or 150/37.5/200 mg tablets, n = 46) or LC (100/25 mg tablets, n = 49), at the same levodopa dose that were administered before randomization. Treatment with LCE resulted in significantly greater improvement in UPDRS part II (ADLs) scores compared to treatment with LC (adjusted mean difference between groups of −1.5 points) (p = 0.0288). Amelioration was also observed in UPDRS part III scores (p = 0.010), and CGI (patient and investigator) scores (p = 0.015, and p = 0.028, respectively). LCE and LC were generally well tolerated with 78 % of subjects completing the study. Most AEs (50 % in LCE and 71.4 % in LC) were classified as mild. No serious AEs were related to the treatment. Treatment with LCE results in improved efficacy compared to LC in PD patients with mild, or minimally disabling motor fluctuations, maintaining a good safety and tolerability profile.







Similar content being viewed by others
References
Boiko AN, Batysheva TT, Minaeva NG et al (2008) Use of the new levodopa agent Stalevo (levodopa/carbidopa/entacapone) in the treatment of Parkinson’s disease in out-patient clinical practice (the START-M open trial). Neurosci Behav Physiol 38:933–936
Brooks DJ (2008) Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat 4:39–47
Eggert K, Skogar O, Amar K et al (2010) Direct switch from levodopa/benserazide or levodopa/carbidopa to levodopa/carbidopa/entacapone in Parkinson’s disease patients with wearing-off: efficacy, safety and feasibility—an open-label, 6-week study. J Neural Transm 117:333–342
Fahn S (2005) Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol 252(Suppl 4):IV37–IV42
Fahn S, Oakes D, Shoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Fung VS, Herawati L, Wan Y (2009) Quality of life in early Parkinson’s disease treated with levodopa/carbidopa/entacapone. Mov Disord 24:25–31
Gordin A, Kaakkola S, Teravainen H (2004) Clinical advantages of COMT inhibition with entacapone—a review. J Neural Transm 111:1343–1363
Hauser RA, Panisset M, Abbruzzese G et al (2009) Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson’s disease. Mov Disord 24:541–550
Larsen JP, Worm-Petersen J, Siden A et al (2003) The tolerability and efficacy of entacapone over 3 years in patients with Parkinson’s disease. Eur J Neurol 10:137–146
Martinez-Martin P, Tolosa E, Hernandez B et al (2008) Validation of the “QUICK” questionnaire–a tool for diagnosis of “wearing-off” in patients with Parkinson’s disease. Mov Disord 23:830–836
Nutt JG, Woodward WR, Beckner RM et al (1994) Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in parkinsonian patients. Neurology 44:913–919
Parkinson Study Group (1997) Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Parkinson Study Group. Ann Neurol 42:747–755
Piccini P, Brooks DJ, Korpela K et al (2000) The catechol-O-methyltransferase (COMT) inhibitor entacapone enhances the pharmacokinetic and clinical response to Sinemet CR in Parkinson’s disease. J Neurol Neurosurg Psychiatry 68:589–594
Reichmann H, Boas J, Macmahon D et al (2005) Efficacy of combining levodopa with entacapone on quality of life and activities of daily living in patients experiencing wearing-off type fluctuations. Acta Neurol Scand 111:21–28
Rinne UK, Larsen JP, Siden A et al (1998) Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 51:1309–1314
Ruottinen HM, Rinne UK (1998) COMT inhibition in the treatment of Parkinson’s disease. J Neurol 245:P25–P34
Schrag A (2005) Entacapone in the treatment of Parkinson’s disease. Lancet Neurol 4:366–370
Solla P, Cannas A, Marrosu F et al (2010) Therapeutic interventions and adjustments in the management of Parkinson disease: role of combined carbidopa/levodopa/entacapone (Stalevo). Neuropsychiatr Dis Treat 6:483–490
Stocchi F (2006) The levodopa wearing-off phenomenon in Parkinson’s disease: pharmacokinetic considerations. Expert Opin Pharmacother 7:1399–1407
Stocchi F, Jenner P, Obeso JA (2010a) When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol 63:257–266
Stocchi F, Rascol O, Kieburtz K et al (2010b) Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol 68:18–27
Yahr MD, Duvoisin RC, Schear MJ et al (1969) Treatment of parkinsonism with levodopa. Arch Neurol 21:343–354
Acknowledgments
This study was supported by Novartis, S.A. Editorial assistance was supported by Montserrat Sabaté and biostatistics by Emma Albacar from Trial Form Support.
This study has been conducted by the above-signed authors and the DERBI study group (in alphabetical order): Adolfo Minguez, Àngels Bayés, Carlos Leiva, Ernest Balaguer, Esther Cubo, Francesc Miquel, Francisco Vivancos, Jose Juni, Juan Andrés Bruguera, Lydia Vela, Matilde Calopa, Maria José Catalán, Mariateresa Buongiorno, Miguel Aguilar, and Oriol de Fàbregues.
Conflict of interest
Hernández B is employee of Novartis S.A. This study was supported by Novartis, S.A.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the DERBI study group.
Rights and permissions
About this article
Cite this article
Tolosa, E., Hernández, B., Linazasoro, G. et al. Efficacy of levodopa/carbidopa/entacapone versus levodopa/carbidopa in patients with early Parkinson’s disease experiencing mild wearing-off: a randomised, double-blind trial. J Neural Transm 121, 357–366 (2014). https://doi.org/10.1007/s00702-013-1114-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1114-x