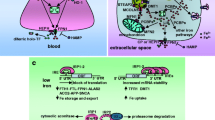
Abstract
A number of neurodegenerative diseases are associated with iron dyshomeostasis and mitochondrial dysfunction. However, the pathomechanistic interplay between iron and mitochondria varies. This review summarises the physiological role of iron in mitochondria and subsequently exemplifies two neurodegenerative diseases with disturbed iron function in mitochondria: inherited Friedreich ataxia (FRDA) and idiopathic Parkinson disease (PD). In eukaryotes, mitochondria are main consumers of iron. The respiratory chain relies on iron-containing redox systems in the form of complexes I–III with iron–sulphur clusters and cytochromes with haem as prosthetic groups. The bifunctional enzyme aconitase is not only important in the citric acid cycle, but also functions as a key regulator of cell iron metabolism. Haem biosynthesis occurs partially in mitochondria as well as the biogenesis of iron–sulphur clusters that are co-factors in numerous iron–sulphur proteins. FRDA is characterised by a mutation of the frataxin gene, the protein of which serves as an iron chaperone in iron–sulphur cluster assembly. The lack of frataxin expression leads to defective iron–sulphur cluster biogenesis with decreased respiratory and aconitase activity. The resulting mitochondrial iron overload might fuel reactive oxygen species formation and contribute to clinical signs of oxidative stress. PD is typically associated with an increased iron content of the substantia nigra, the causes of which are largely unknown. Recent research demonstrated raised iron levels in individual dopaminergic neurons of the substantia nigra. Moreover, transferrin/transferrin receptor 2 mediated transport of iron into the mitochondria of these neurons was identified together with increased transferrin immunoreactivity. Resulting accumulation of iron into mitochondria might lead to oxidative stress damaging iron–sulphur cluster-containing proteins.




Similar content being viewed by others
References
Acquaviva F, De Biase, I, Nezi L, Ruggiero G, Tatangelo F, Pisano C, Monticelli A, Garbi C, Acquaviva AM, Cocozza S (2005) Extra-mitochondrial localisation of frataxin and its association with IscU1 during enterocyte-like differentiation of the human colon adenocarcinoma cell line Caco-2. J Cell Sci 118:3917–3924
Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B (1997) A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem 69:1326–1329
Ambani LM, Van Woert MH, Murphy S (1975) Brain peroxidase and catalase in Parkinson disease. Arch Neurol 32:114–118
Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, Bishop DF (2000) Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron–sulfur protein maturation. Blood 96:3256–3264
Berg D (2007) Disturbance of iron metabolism as a contributing factor to SN hyperechogenicity in Parkinson’s disease: implications for idiopathic and monogenetic forms. Neurochem Res 32:1646–1654
Berg D, Siefker C, Becker G (2001) Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol 248:684–689
Bidichandani SI, Ashizawa T, Patel PI (1998) The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet 62:111–121
Blat D, Weiner L, Youdim MB, Fridkin M (2008) A Novel iron-chelating derivative of the neuroprotective peptide NAPVSIPQ shows superior antioxidant and antineurodegenerative capabilities. J Med Chem 51:126–134
Borie C, Gasparini F, Verpillat P, Bonnet AM, Agid Y, Hetet G, Brice A, Durr A, Grandchamp B (2002) Association study between iron-related genes polymorphisms and Parkinson’s disease. J Neurol 249:801–804
Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH (2000) Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet 9:275–282
Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, Levi S, Iolascon A (2007) The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood 110:1353–1358
Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427
Cavadini P, Biasiotto G, Poli M, Levi S, Verardi R, Zanella I, Derosas M, Ingrassia R, Corrado M, Arosio P (2007) RNA silencing of the mitochondrial ABCB7 transporter in HeLa cells causes an iron-deficient phenotype with mitochondrial iron overload. Blood 109:3552–3559
Condo I, Ventura N, Malisan F, Tomassini B, Testi R (2006) A pool of extramitochondrial frataxin that promotes cell survival. J Biol Chem 281:16750–16756
Delatycki MB, Williamson R, Forrest SM (2000) Friedreich ataxia: an overview. J Med Genet 37:1–8
Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989a) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52:381–389
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989b) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52:1830–1836
Emond M, Lepage G, Vanasse M, Pandolfo M (2000) Increased levels of plasma malondialdehyde in Friedreich ataxia. Neurology 55:1752–1753
Gakh O, Park S, Liu G, Macomber L, Imlay JA, Ferreira GC, Isaya G (2006) Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Hum Mol Genet 15:467–479
Gandhi S, Wood NW (2005) Molecular pathogenesis of Parkinson’s disease. Hum Mol Genet 14:2749–2755
Gille G, Reichmann H (2009) Mitochondriale Störungen beim Parkinson-Syndrom. Nervenheilkunde 28:281–288
Gonzalez-Cabo P, Vazquez-Manrique RP, Garcia-Gimeno MA, Sanz P, Palau F (2005) Frataxin interacts functionally with mitochondrial electron transport chain proteins. Hum Mol Genet 14:2091–2098
Grabczyk E, Usdin K (2000) The GAA*TTC triplet repeat expanded in Friedreich’s ataxia impedes transcription elongation by T7 RNA polymerase in a length and supercoil dependent manner. Nucl Acids Res 28:2815–2822
Griffiths PD, Dobson BR, Jones GR, Clarke DT (1999) Iron in the basal ganglia in Parkinson’s disease. An in vitro study using extended X-ray absorption fine structure and cryo-electron microscopy. Brain 122:667–673
Guo B, Yu Y, Leibold EA (1994) Iron regulates cytoplasmic levels of a novel iron-responsive element-binding protein without aconitase activity. J Biol Chem 269:24252–24260
Haller RG, Henriksson KG, Jorfeldt L, Hultman E, Wibom R, Sahlin K, Areskog NH, Gunder M, Ayyad K, Blomqvist CG (1991) Deficiency of skeletal muscle succinate dehydrogenase and aconitase. Pathophysiology of exercise in a novel human muscle oxidative defect. J Clin Invest 88:1197–1206
Heinemann IU, Jahn M, Jahn D (2008) The biochemistry of heme biosynthesis. Arch Biochem Biophys 474:238–251
Iwai K, Klausner RD, Rouault TA (1995) Requirements for iron-regulated degradation of the RNA binding protein, iron regulatory protein 2. EMBO J 14:5350–5357
Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zochling R, Boissl KW, Reichmann H (1994) Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci Lett 169:126–128
Jenner P, Olanow CW (2006) The pathogenesis of cell death in Parkinson’s disease. Neurology 66:S24–S36
Kakhlon O, Breuer W, Munnich A, Cabantchik ZI (2010) Iron redistribution as a therapeutic strategy for treating diseases of localized iron accumulation. Can J Physiol Pharmacol 88:187–196
Kennedy MC, Emptage MH, Dreyer JL, Beinert H (1983) The role of iron in the activation-inactivation of aconitase. J Biol Chem 258:11098–11105
Kerr DS (2010) Treatment of mitochondrial electron transport chain disorders: a review of clinical trials over the past decade. Mol Genet Metab 99:246–255
Klausner RD, Rouault TA, Harford JB (1993) Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72:19–28
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520
Larsson LE, Linderholm H, Mueller R, Ringqvist TL, Soernaes R (1964) Hereditary metabolic myopathy with paroxysmal myoglobinuria due to abnormal glycolysis. J Neurol Neurosurg Psychiatr 27:361–380
Lee DW, Kaur D, Chinta SJ, Rajagopalan S, Andersen JK (2009) A disruption in iron–sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson’s disease. Antioxid Redox Signal 11:2083–2094
Lill R (2009) Function and biogenesis of iron–sulphur proteins. Nature 460:831–838
Lill R, Muhlenhoff U (2008) Maturation of iron–sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem 77:669–700
Lill R, Dutkiewicz R, Elsasser HP, Hausmann A, Netz DJ, Pierik AJ, Stehling O, Urzica E, Muhlenhoff U (2006) Mechanisms of iron–sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes. Biochim Biophys Acta 1763:652–667
Lodi R, Cooper JM, Bradley JL, Manners D, Styles P, Taylor DJ, Schapira AH (1999) Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc Natl Acad Sci USA 96:11492–11495
Marttila RJ, Lorentz H, Rinne UK (1988) Oxygen toxicity protecting enzymes in Parkinson’s disease. Increase of superoxide dismutase-like activity in the substantia nigra and basal nucleus. J Neurol Sci 86:321–331
Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, Greenamyre JT (2009) A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol Dis 34:417–431
Melefors O, Hentze MW (1993) Iron regulatory factor—the conductor of cellular iron regulation. Blood Rev 7:251–258
Mochel F, Knight MA, Tong WH, Hernandez D, Ayyad K, Taivassalo T, Andersen PM, Singleton A, Rouault TA, Fischbeck KH, Haller RG (2008) Splice mutation in the iron–sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. Am J Hum Genet 82:652–660
Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM (2007) Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68:1820–1825
Olsson A, Lind L, Thornell LE, Holmberg M (2008) Myopathy with lactic acidosis is linked to chromosome 12q23.3–24.11 and caused by an intron mutation in the ISCU gene resulting in a splicing defect. Hum Mol Genet 17:1666–1672
Pagon RA, Bird TD, Detter JC, Pierce I (1985) Hereditary sideroblastic anaemia and ataxia: an X linked recessive disorder. J Med Genet 22:267–273
Pandolfo M (2002) The molecular basis of Friedreich ataxia. Adv Exp Med Biol 516:99–118
Pandolfo M (2008) Friedreich ataxia. Arch Neurol 65:1296–1303
Paraskeva E, Hentze MW (1996) Iron–sulphur clusters as genetic regulatory switches: the bifunctional iron regulatory protein-1. FEBS Lett 389:40–43
Piemonte F, Pastore A, Tozzi G, Tagliacozzi D, Santorelli FM, Carrozzo R, Casali C, Damiano M, Federici G, Bertini E (2001) Glutathione in blood of patients with Friedreich’s ataxia. Eur J Clin Invest 31:1007–1011
Reichmann H, Riederer P, Seufert S, Jellinger K (1990) Disturbances of the respiratory chain in brain from patients with Parkinson’s disease. Mov Disord 5(Suppl 1):28
Reichmann H, Janetzky B, Riederer P (1995) Iron-dependent enzymes in Parkinson’s disease. J Neural Transm Suppl 46:157–164
Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52:515–520
Rötig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P (1997) Aconitase and mitochondrial iron–sulphur protein deficiency in Friedreich ataxia. Nat Genet 17:215–217
Rouault TA, Tong WH (2005) Opinion: iron–sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol 6:345–351
Rouault TA, Tong WH (2008) Iron–sulfur cluster biogenesis and human disease. Trends Genet 24:398–407
Saggu H, Cooksey J, Dexter D, Wells FR, Lees A, Jenner P, Marsden CD (1989) A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem 53:692–697
Sakamoto N, Ohshima K, Montermini L, Pandolfo M, Wells RD (2001) Sticky DNA, a self-associated complex formed at long GAA·TTC repeats in intron 1 of the frataxin gene, inhibits transcription. J Biol Chem 276:27171–27177
Sanchez-Ramos JR, Övervik E, Ames BN (1994) A marker of oxyradical-mediated DNA damage (8-hydroxy-2′deoxyguanosine) is increased in nigro-striatum of Parkinson’s disease brain. Neurodegeneration 3:197–204
Saveliev A, Everett C, Sharpe T, Webster Z, Festenstein R (2003) DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nature 422:909–913
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 333:1269
Schoenfeld RA, Napoli E, Wong A, Zhan S, Reutenauer L, Morin D, Buckpitt AR, Taroni F, Lonnerdal B, Ristow M, Puccio H, Cortopassi GA (2005) Frataxin deficiency alters heme pathway transcripts and decreases mitochondrial heme metabolites in mammalian cells. Hum Mol Genet 14:3787–3799
Schulz JB, Dehmer T, Schols L, Mende H, Hardt C, Vorgerd M, Burk K, Matson W, Dichgans J, Beal MF, Bogdanov MB (2000) Oxidative stress in patients with Friedreich ataxia. Neurology 55:1719–1721
Shoichet SA, Baumer AT, Stamenkovic D, Sauer H, Pfeiffer AF, Kahn CR, Muller-Wieland D, Richter C, Ristow M (2002) Frataxin promotes antioxidant defense in a thiol-dependent manner resulting in diminished malignant transformation in vitro. Hum Mol Genet 11:815–821
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Tong WH, Rouault TA (2006) Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron–sulfur cluster biogenesis and iron homeostasis. Cell Metab 3:199–210
Tozzi G, Nuccetelli M, Lo BM, Bernardini S, Bellincampi L, Ballerini S, Gaeta LM, Casali C, Pastore A, Federici G, Bertini E, Piemonte F (2002) Antioxidant enzymes in blood of patients with Friedreich’s ataxia. Arch Dis Child 86:376–379
von Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, Oertel W, Siebert U, Berger K, Dodel R (2005) Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol 15:473–490
Weinreb O, Amit T, Mandel SA, Kupershmidt L, Youdim MB (2010) Neuroprotective multifunctional iron chelators: from redox-sensitive process to novel therapeutic opportunities. Antioxid Redox Signal 13:919–949
Weiss G, Wachter H, Fuchs D (1995) Linkage of cell-mediated immunity to iron metabolism. Immunol Today 16:495–500
Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, Rouault TA (2010) Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J Clin Invest 120:1749–1761
Yoon T, Cowan JA (2004) Frataxin-mediated iron delivery to ferrochelatase in the final step of heme biosynthesis. J Biol Chem 279:25943–25946
Zecca L, Tampellini D, Gatti A, Crippa R, Eisner M, Sulzer D, Ito S, Fariello R, Gallorini M (2002) The neuromelanin of human substantia nigra and its interaction with metals. J Neural Transm 109:663–672
Zucca FA, Giaveri G, Gallorini M, Albertini A, Toscani M, Pezzoli G, Lucius R, Wilms H, Sulzer D, Ito S, Wakamatsu K, Zecca L (2004) The neuromelanin of human substantia nigra: physiological and pathogenic aspects. Pigment Cell Res 17:610–617
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gille, G., Reichmann, H. Iron-dependent functions of mitochondria—relation to neurodegeneration. J Neural Transm 118, 349–359 (2011). https://doi.org/10.1007/s00702-010-0503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0503-7