Abstract
Background
Efficacy, safety and pharmacokinetics of simeprevir (TMC435), a once-daily, noncovalent, oral hepatitis C virus (HCV) NS3/4A protease inhibitor, was evaluated in combination with peginterferon α-2a/ribavirin (PegIFNα-2a/RBV) for treatment-naïve, HCV genotype 1-infected patients in Japan.
Methods
In a multicenter, randomized clinical trial in Japan, ninety-two patients received either simeprevir (50 or 100 mg QD) for 12 or 24 weeks with PegIFNα-2a/RBV for 24 or 48 weeks (according to response-guided therapy [RGT] criteria), or PegIFNα-2a/RBV for 48 weeks (PR48 group).
Results
Compared with the PR48 group, plasma HCV RNA reductions in the simeprevir groups were rapid and more substantial (Week 4: −5.2, −5.2 and −2.9 log10IU/mL for simeprevir 50 mg combined, 100 mg combined, and PR48 groups, respectively). High rapid virologic response rates (83, 90, and 8 % for simeprevir 50 mg combined, 100 mg combined, and PR48 groups, respectively) led to high sustained virologic response rates (77–92 %, compared with 46 % for PR48). All but one of the simeprevir-treated patients were eligible to complete treatment after 24 weeks (RGT). Relapse rates in simeprevir-treated patients were low (8–17 %, compared with 36 % for the PR48 group). There were no notable differences in the safety profile between the simeprevir and PR48 groups.
Conclusions
The addition of simeprevir QD to PegIFNα-2a/RBV, as compared with PegIFNα-2a/RBV alone, demonstrated potent antiviral activity and significantly improved the rates of sustained virologic response, with a shortened 24-week treatment duration, in treatment-naive patients infected with HCV genotype 1 in Japan. Simeprevir was generally safe and well tolerated. (ClinicalTrials.gov number, NCT00996476).
Similar content being viewed by others
Introduction
The hepatitis C virus (HCV) is a leading cause of chronic liver disease worldwide infecting an estimated 130–170 million people, or 2.2–3.0 % of the global population [1, 2]. In Japan, hepatocellular carcinoma (HCC) is one of the most common causes of cancer mortality (incidence of 7 % in Japan, compared with 1–4 % in Europe and the USA [3]) and 79 % of HCC cases are due to HCV infection [4, 5]. An estimated 2 million Japanese people are infected, 70 % with HCV genotype 1b, 20 % with genotype 2a, and the remainder with genotype 2b or other genotypes [6].
Until recently, the standard of care for the treatment of chronic genotype 1 HCV infection was a combination of peginterferon (PegIFNα-2a) and ribavirin (RBV) for 48 weeks or longer [2, 7–9]. However, the long treatment duration is a substantial physical and mental burden for patients, and rates of treatment discontinuation and dose reduction due to adverse events (AEs) are high [10, 11]. Furthermore, sustained virologic response (SVR, defined as undetectable HCV RNA at a given time point after the end of treatment) is achieved in only 42–52 % of patients [10–12]. Novel, direct-acting antiviral agents (DAAs) are therefore under development. Two first-generation HCV NS3/4A protease inhibitors (PIs), boceprevir [13, 14] and telaprevir [15, 16], have recently been approved for the treatment of genotype 1-infected patients in the USA and Europe, and telaprevir is also approved in Japan [17, 18]. The inclusion of these agents in HCV treatment regimens has led to large improvements in SVR rates, though these agents require dosing three times daily (every 7-9 h) and their use is associated with increased incidence and, in some cases, severity of AEs such as anemia and rash [13, 16, 18–21].
Simeprevir (TMC435) is an investigational, once-daily (QD) oral NS3/4A protease inhibitor currently under clinical development globally. International Phase IIb trials of simeprevir in combination with PegIFNα-2a/RBV for treatment-naïve and -experienced patients infected with HCV genotype 1 demonstrated that simeprevir was generally well tolerated, had a pharmacokinetic profile that supports QD dosing and resulted in high virologic response rates [22–25].
In a Phase I study in 30 healthy Japanese adult male volunteers living in the USA (TMC435-C109; NCT00752544), simeprevir was generally well tolerated. Simeprevir plasma exposures were higher in Japanese healthy volunteers compared with Caucasian volunteers in the TMC435-C101 study (NCT00938899) [26].
The Dose and duration Ranging study of Antiviral agent TMC435 in Genotype One HCV treatment-Naïve patients (DRAGON; TMC435-C215) was a Phase II study conducted across Japan to evaluate the efficacy, safety and pharmacokinetics of simeprevir and PegIFNα-2a/RBV in treatment-naïve, HCV-infected patients. Based on the higher exposure of simeprevir in Japanese versus Caucasian healthy volunteers demonstrated in the Phase I study, simeprevir doses of 50 and 100 mg QD were selected for evaluation in this dose-ranging, Japanese Phase II study.
Methods
Patients
Patients recruited to the DRAGON study were treatment-naïve, chronically infected with genotype 1 HCV, aged 20–70 years and had plasma levels of HCV RNA ≥5.0 log10 IU/mL at screening.
Key exclusion criteria included: (1) presence of liver cirrhosis or hepatic failure, or other liver disease, (2) infection/co-infection with HIV-1, HIV-2, hepatitis B or nongenotype 1 HCV, (3) malignant tumor within 5 years prior to study, (4) HCC, (5) meeting conditions that required caution with PegIFNα-2a or RBV treatment, (6) any clinically significant disease, (7) organ transplant, and (8) defined laboratory abnormalities during screening.
Study design
The DRAGON study was a multicenter, randomized, open-label, parallel group comparison study performed to evaluate the efficacy, safety and pharmacokinetics of simeprevir in combination with PegIFNα-2a/RBV in treatment-naïve patients with chronic genotype 1 HCV infection. The study was performed from July 6, 2009, to April 1, 2011, in accordance with the Declaration of Helsinki, and was consistent with Good Clinical Practice guidelines and applicable regulatory requirements. The study protocol and amendments were reviewed by Institutional Review Boards and each patient provided written informed consent.
Eligible patients were randomized to one of five treatment groups (SMV12/PR24 50 mg, SMV24/PR24 50 mg, SMV12/PR24 100 mg, SMV24/PR24 100 mg and PR48) in a 2:1:2:1:1 ratio. In the SMV12/PR24 50 mg and SMV12/PR24 100 mg groups, patients received simeprevir (50 or 100 mg QD, respectively) combined with PegIFNα-2a/RBV for 12 weeks, followed by PegIFNα-2a/RBV for 12 weeks. In the SMV24/PR24 50 mg and SMV24/PR24 100 mg groups, patients received simeprevir (50 or 100 mg QD, respectively) combined with PegIFNα-2a/RBV for 24 weeks. In these four groups, at week 24, patients either stopped or continued treatment with PegIFNα-2a/RBV up to week 48, according to response-guided therapy (RGT) criteria (stop treatment if plasma HCV RNA <1.4 log10 IU/mL at week 4 and undetectable at weeks 12, 16 and 20, otherwise continuing PegIFNα-2a/RBV to week 48). In the PR48 group, criteria were not applied; patients received PegIFNα-2a/RBV for 48 weeks.
Major efficacy endpoints included the proportion of patients with undetectable plasma HCV RNA 24 weeks after the end of treatment (SVR24). Other efficacy endpoints included the proportion of patients with undetectable plasma HCV RNA at week 4 (rapid virologic response; RVR) or week 12 (complete early virologic response, cEVR) and change in plasma HCV RNA level from baseline to week 4 of treatment. Incidence of viral breakthrough (increase of >1 log10 IU/mL in plasma HCV RNA level from the lowest level reached, or plasma HCV RNA level of >2.0 log10 IU/mL in patients whose plasma HCV RNA level had previously been below 1.4 log10 IU/mL or undetectable), viral relapse (detectable plasma HCV RNA during the post-treatment follow-up period in patients who had undetectable plasma HCV RNA at the end of treatment) and the viral NS3 sequence were also assessed.
According to predefined virologic stopping rules, patients in the simeprevir groups discontinued simeprevir and continued PegIFNα-2a/RBV if viral breakthrough occurred during the first 24 weeks, and stopped all treatment if the decrease in plasma HCV RNA from baseline to week 12 was <2 log10 IU/mL, or plasma HCV RNA level at week 24 was ≥1.2 log10 IU/mL.
The population-based sequencing assay to determine HCV NS3/4A sequence based on the standard Sanger sequencing method was performed for patients with viral breakthrough and viral relapse. Sequencing data were reported as amino acid changes from Con1 (GenBank accession number AJ238799) or H77 (GenBank accession number AF009606) for genotype 1b and 1a/others, respectively.
Safety endpoints included the frequency and severity of AEs including serious AEs (SAEs), incidence of discontinuation of study drugs due to AEs, and changes in clinical laboratory test values.
Treatment administration
Simeprevir was administered orally at a dose of 50 or 100 mg as a single capsule QD. No simeprevir dose reduction was permitted. PegIFNα-2a (Pegasys®, Chugai) was administered as a subcutaneous injection (180 μg once weekly) and RBV (Copegus®, Chugai) as oral tablets (600–1000 mg total daily dose, depending on body weight) in accordance with the manufacturer’s prescribing information for both medications. Patients were hospitalized for a minimum of 1 week, starting on the first day that simeprevir and PegIFNα-2a/RBV were administered. Treatment of anemia with erythropoietin was not permitted in the study.
Measurements
Plasma HCV RNA was quantified upon screening, at baseline, at 4 and 8 h on day 1, on day 3, at weeks 1, 2, 3, 4, 6, 8, 12, 16, 20 and 24 (all patients), and weeks 28, 36 and 48 (patients stopping PegIFNα-2a/RBV at week 24) or weeks 28, 36, 42, 48, 52, 60 and 72 (patients receiving PegIFNα-2a/RBV until week 48), using the Roche COBAS® TaqMan® HCV Auto assay system (lower limit of quantification [LLOQ] 15 IU/mL, equivalent to 1.2 log10 IU/mL). Plasma HCV RNA below the LLOQ was either assigned as ‘HCV RNA <1.2 log10 IU/mL detectable’ if traces of HCV RNA were detected, or ‘undetectable HCV RNA’ if no HCV RNA was detected. Alanine aminotransferase (ALT) was quantified at baseline and weeks 1, 2, 4, 6, 8, 12, 16, 20 and 24 (all patients), and weeks 28 and 48 (patients stopping at week 24), or weeks 36, 42, 48, 52 and 72 (patients receiving PegIFNα-2a/RBV until week 48).
Laboratory test values, results of vital sign assessment and presence or absence of electrocardiogram abnormalities were recorded at screening and at regular intervals throughout treatment and the post-treatment follow-up period. AEs were recorded throughout the study period.
Statistical analysis
Efficacy analyses were performed on the full analysis set (FAS; all randomized patients with post-baseline efficacy assessment data). For virologic responses (RVR, cEVR and SVR24), rates were summarized for the combined simeprevir 50 mg (i.e. SMV12/PR24 50 mg and SMV24/PR24 50 mg groups pooled) and 100 mg (i.e. SMV12/PR24 100 mg and SMV24/PR24 100 mg groups pooled) groups, and compared with that of the PR48 group. In addition, for SVR24, rates were calculated for each of the five treatment groups. The change in HCV RNA from baseline to week 4 was assessed using analysis of covariance (ANCOVA), with the dose group as a factor and plasma HCV RNA at baseline as a covariate, to calculate the least-squares (LS) means and two-tailed corresponding 95 % confidence intervals (CIs). These were calculated for change from baseline for the combined simeprevir 50 mg or combined simeprevir 100 mg groups and the PR48 group, and the differences between groups. Fisher’s exact test was performed to compare RVR and cEVR between the combined simeprevir groups and the PR48 group.
A mutation was considered as emerging at a specific time point if the amino acid at a given position was absent at baseline and present at that time point. Incidence of emergent mutations at the time of viral breakthrough or relapse was summarized.
The proportion of patients displaying ALT within the normal limits was summarized for all patients in the combined simeprevir groups and for the PR48 group. Incidence of AEs and other safety endpoints were analyzed for all patients who received at least one dose of medication and were summarized per treatment group.
Results
Patient disposition and baseline characteristics
In total, 116 patients were screened and 93 were randomized to treatment groups, of whom 92 received at least one dose of a study drug (Supplementary Fig. 1). Ninety-one percent of the patients completed the study. The main reasons for study discontinuation were withdrawal of consent (5 %) and occurrence of AEs (2 %). Out of seven patients who discontinued from the study during the post-treatment follow-up period, five patients dropped out due to withdrawal of consent, and two (1 in SMV12/PR24 100 mg group, 1 in PR48 group) due to AEs.
In the simeprevir groups, nine patients permanently discontinued all treatment due to AEs (8/79, 10 %) or virologic stopping criteria (1/79, 1.3 %). In the PR48 group, three patients permanently discontinued all treatment due to AEs (n = 2) or another reason (n = 1).
There were no notable differences in baseline demographic characteristics between treatment groups (Table 1). Approximately half of the patients (39 to 62 %) were male, with a median age of 54 years (range 20–69 years) and median body weight of 58 kg (range 40–85 kg). All patients were infected with HCV genotype 1b and the median baseline HCV RNA was 6.3 log10 IU/mL (range 4.5–7.0 log10 IU/mL). A total of 7 % of patients displayed Metavir fibrosis stage 3; there were no patients with fibrosis stage 4.
Efficacy
On-treatment virologic response
During the first 3–7 days of simeprevir administration, an initial rapid reduction in plasma HCV RNA was evident in all simeprevir groups (Fig. 1). For analysis of the impact of simeprevir dose with regards to on-treatment virologic response, data were pooled by dose group (simeprevir 50 mg groups combined and 100 mg groups combined; Table 2).
The mean HCV RNA change from baseline to week 4 was significantly greater in the simeprevir combined 50 and 100 mg groups than in the PR48 group; LS mean differences from the PR48 group were 2.4 for both simeprevir 50 mg combined and 100 mg combined [both of the least-squares mean difference values (95 % confidence interval) were below zero].
The majority of patients in the simeprevir 50 mg combined and 100 mg combined groups achieved RVR and cEVR (83–90 and 92–98 %, respectively), compared with rates of 8 and 54 %, respectively, in the PR48 group.
Sustained virologic response
The SVR24 rate was higher in the simeprevir groups than in the PR48 group, with rates of 78, 77, 77, 92 and 46 in the SMV12/PR24 50 mg, SMV24/PR24 50 mg, SMV12/PR24 100 mg, SMV24/PR24 100 mg and PR48 groups, respectively (Table 2; Fig. 2).
All patients in the simeprevir groups, except for one patient in the SMV12/PR24 100 mg group, successfully completed treatment at week 24, as they met the RGT criteria. Among these patients, SVR24 rates ranged from 83 to 90 %.
All seven patients who discontinued the study during the follow-up period were classified as non-SVR, although four out of the seven patients had undetectable HCV RNA at the last visit before withdrawal.
Viral breakthrough, viral relapse or treatment failure
One patient in the SMV12/PR24 50 mg group experienced viral breakthrough at week 20 after the HCV RNA level became undetectable from weeks 3 to 16. This patient had viral breakthrough during treatment with PegIFNα-2a/RBV, after the completion of triple therapy. No viral breakthrough was reported in the other simeprevir groups or the PR48 group.
Compared to the PR48 group (36 %), viral relapse rates were apparently lower in the simeprevir groups regardless of dose or duration (15, 17, 15 and 8 % in the SMV12/PR24 50 mg, SMV24/PR24 50 mg, SMV12/PR24 100 mg, and SMV24/PR24 100 mg group, respectively) (Table 2).
Among the patients in the simeprevir treatment groups, 20 % (16/79) did not achieve SVR24. The reasons for this were detectable HCV RNA at the end of treatment (two patients), viral relapse (11 patients), or missing HCV RNA results for patients at 24 weeks after they had achieved undetectable levels at the end of treatment (three patients).
Viral population sequencing
No emerging mutations were observed in the selected HCV NS3 protease domain in the patient with viral breakthrough. Paired HCV NS3 sequence information (at baseline and time of relapse) was available for 10/11 simeprevir-treated relapsers, and emerging mutations in the NS3 protease domain (Q80R, R155Q, D169A, C, E, H and/or V, alone or in combination) were detected in six of these ten patients.
Alanine aminotransferase
The proportion of simeprevir-treated patients who had ALT levels within the normal limit had increased from 57 % at baseline to 92 % at the end of treatment (per protocol analysis).
Safety
The rates of simeprevir and PegIFNα-2a/RBV discontinuation and PegIFNα-2a/RBV dose modification due to AEs were similar between the simeprevir groups and the PR48 group.
There was one death reported due to cerebral infarction in the SMV12/PR24 100 mg group. The patient was a 64-year-old female with hypertension but no other notable medical history. The death occurred approximately 3 weeks after the end of treatment and was therefore considered to be unrelated to simeprevir and PegIFNα-2a/RBV.
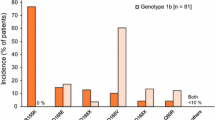
A summary of AEs is shown in Table 3. There were no clinically relevant differences in the incidence of AEs across the groups and the majority of AEs were of grade 1 or 2 in severity according to the WHO toxicity grading predefined in the study protocol. Rash and arthralgia, which were slightly higher (>15 % numerical difference) in the simeprevir groups than in the PR48 group, were also grade 1 or 2 in severity.
For protocol-predefined rash/cutaneous reactions (except for pruritus without visible skin findings) considered by the investigator to be caused by any medication, the incidence rate was similar between the simeprevir groups and the PR48 group, with no notable differences across the simeprevir groups and with no grade 3 or 4 events, such as Stevens-Johnson syndrome or toxic epidermal necrosis, reported (Table 3). As for pruritus, which was reported in 19 % of the simeprevir-treated patients (compared to none in the PR group), all events were grade 1 or 2 in severity.
Incidences of anemia and decreased hemoglobin were similar in the simeprevir groups and the PR48 group, as were the rates of discontinuation and RBV dose reduction due to these two AEs. All were grade 1 or 2 and no notable differences were reported across the simeprevir groups. Of note, treatment with erythropoietin was not permitted in the study. Changes in hemoglobin levels from baseline were also similar across the groups and none of the patients had values <8 g/dL (Fig. 3a).
Mild and transient increases in bilirubin levels (direct and indirect) were observed in the simeprevir groups during the first 2 weeks. Mean bilirubin values stabilized or decreased during continued treatment and returned to baseline values after week 12 in the SMV12/PR24 groups and week 24 in the SMV24/PR24 groups (Fig. 3b). These changes were not associated with increases in the laboratory parameters for ALT or aspartate aminotransferase. Four simeprevir-treated patients experienced grade 3 (2.6–5.0 mg/dL) or 4 (>5.0 mg/dL) total bilirubin elevations during weeks 1 to 2 of treatment (the ratio direct/total bilirubin at the time point of the highest bilirubin value in these four patients were 26.5, 31.6, 60.7 and 17.5 %, respectively) and discontinued simeprevir in accordance with the treatment discontinuation criteria, which were predefined in the study protocol. All of these bilirubin elevations began to decrease before or immediately after the end of simeprevir treatment and returned to baseline after the end of simeprevir treatment. No particular changes (elevations) in other hepatic parameters or any clinically relevant symptoms were reported in these patients. No other clinically significant differences in laboratory parameters between the simeprevir groups and the PR48 group were observed.
Discussion
This study was the first in treatment-naïve Asian patients chronically infected with HCV genotype 1 and with high viral load, to investigate the efficacy, safety and pharmacokinetics of simeprevir as part of a treatment regimen including PegIFNα-2a and RBV. In this study, all patients were infected with HCV genotype 1b, reflecting the high prevalence of this subtype in Japan.
Results of the study indicated that once-daily simeprevir (50 and 100 mg) in combination with PegIFNα-2a/RBV significantly improved the SVR24 rate compared with PegIFNα-2a/RBV alone in this patient population.
In this study, an RGT strategy was employed to allow individualized shortening of PegIFNα/RBV treatment duration to 24 weeks, based on early viral kinetics. According to the RGT criteria, the majority of simeprevir patients were eligible to stop all treatment at week 24. Furthermore, these patients had high SVR24 rates (83–90 %). This indicates that an RGT approach is also beneficial for patients in Japan. Shorter overall treatment duration is highly desirable as it reduces the length of exposure to PegIFNα-2a/RBV, thereby potentially reducing the duration of AEs experienced by the patient, and is also a cost-effective alternative to standard-duration PegIFNα/RBV therapy [27].
During the first 3 days of dosing, both 50 and 100 mg doses of simeprevir demonstrated an initial rapid reduction in HCV RNA. Subsequent reduction in HCV RNA was less pronounced, and by week 4, the LS mean HCV RNA decline from baseline was −5.2 log10 IU/mL in both the simeprevir 50 mg combined and 100 mg combined groups, compared with −2.9 log10 IU/mL in the PR48 group. This biphasic reduction in HCV RNA was consistent with viral kinetics observed in a previous proof-of-concept simeprevir study (OPERA-1) [23] and other potent DAAs. In all simeprevir groups, RVR was highly predictive of SVR. Following high RVR rates (83–90 %, compared with 8 % in the PR48 group), simeprevir-treated patients achieved high SVR24 rates (77–92 %, compared with 46 % in the PR48 group), accompanied by low rates of viral relapse (8–17 %, compared with 36 % in the PR48 group). The response rates in the PR48 group are consistent with previous reports [18, 22, 28].
No viral breakthrough was reported in the simeprevir 100 mg groups during the treatment period. In addition, the on-treatment virologic response rate in the early treatment phase (weeks 2 to 4) was slightly higher in the simeprevir combined 100 mg group compared with the combined 50 mg group (e.g., 44 % of the patients in the simeprevir 100 mg group had undetectable HCV RNA, versus 28 % in the simeprevir 50 mg group at week 2). The on-treatment response was not affected by the duration of simeprevir treatment across dose group (RVR rate of 85, 77, 89 and 92 %; cEVR rate of 100, 92, 89 and 100 %, in the SMV12/PR24 50 mg, SMV24/PR24 50 mg, SMV12/PR24 100 mg and SMV24/PR24 100 mg groups, respectively), and no clear relationship between relapse rate and duration of simeprevir treatment was observed. Therefore, the simeprevir 100 mg dose with a 12-week duration as triple therapy has been selected to be taken forward into Phase III trials.
In addition to the notable virologic response, the study demonstrated greater increases from baseline to the end of treatment in the proportion of simeprevir-treated patients (per protocol population) with normal ALT levels.
Overall, these results are consistent with previous simeprevir trials which investigated HCV genotype 1-infected, treatment-naïve patients, conducted mainly in the USA and Europe [23].
In simeprevir-treated patients with viral relapse for whom sequence information was available, emerging mutations in the NS3 protease domain (Q80R, R155Q, D168A/C/E/H and V, alone or in combination) were detected around the time of viral relapse in 6/10 patients. Mutations at these positions have been previously identified in vitro [29]. Considering the dominance of the HCV genotype 1b in Japan, further evaluation of emerging mutations is required.
There were no notable differences in incidence of AEs or discontinuations due to AEs between groups receiving triple therapy of simeprevir with PegIFNα-2a/RBV and those receiving PegIFNα-2a/RBV alone. In contrast to the first-generation PIs [13, 16, 19–21], no severe anemia or severe rash/cutaneous reactions (including, though not limited to, Stevens-Johnson syndrome or toxic epidermal necrosis) were reported in this study. Except for bilirubin levels, there were no significant changes in laboratory values including hemoglobin, neutrophil count and platelet count. Mild, transient increases in bilirubin (direct, indirect and total) levels were observed in simeprevir-treated patients, though these were not clinically significant or accompanied by increases in other hepatic function parameters. These bilirubin level elevations were grade 3 (2.6–5.0 mg/dL) or 4 (>5.0 mg/dL) in four simeprevir-treated patients, which led to the discontinuation of simeprevir in these individuals. The bilirubin levels began to decrease before the end of simeprevir treatment and returned to baseline after the end of simeprevir treatment. In vitro studies indicated that this may be due to the inhibition of OATP1B1 and MRP2 transporters by simeprevir, as both have a role in bilirubin clearance [30]. However, it is not anticipated that inhibition of these transporters will affect dosing, efficacy or safety in ongoing studies.
In conclusion, in Japanese treatment-naïve patients infected with HCV genotype 1b with high viral load, treatment with oral, once-daily simeprevir in combination with PegIFNα-2a and RBV, regardless of simeprevir dose regimen (50 or 100 mg QD, for 12 or 24 weeks), demonstrated potent antiviral activity and high SVR rates, and shortened the overall treatment duration in the majority of patients. Simeprevir was well tolerated, with no additional side effects or incremental adverse events. The mild and reversible bilirubin elevation was asymptomatic and not accompanied by elevation of other hepatic parameters.
Novel DAAs are expected to continue the improvement of HCV treatment (i.e., further improved safety profile, more convenient dosing regimens, and reduced resistance), started by the addition of the first generation of protease inhibitors to PegIFN/RBV combination therapy. Based on the data provided by this Phase II DRAGON study, the Phase III CONCERTO trials will shed further light on the treatment of chronic HCV genotype-1 infection in treatment-naïve and -experienced patients in Japan.
Abbreviations
- AE:
-
Adverse event
- ALT:
-
Alanine aminotransferase
- CI:
-
Confidence interval
- DAA:
-
Direct-acting antiviral agent
- DRAGON:
-
The Dose and duration Ranging study of Antiviral agent TMC435 in Genotype One HCV treatment-Naïve patients
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- LS:
-
Least squares
- QD:
-
Once daily
- PegIFNα-2a:
-
Peginterferon α-2a
- PI:
-
Protease inhibitor
- RBV:
-
Ribavirin
- RGT:
-
Response-guided therapy
- RNA:
-
Ribonucleic acid
- SAE:
-
Serious adverse event
- SVR24:
-
Sustained virologic response 24 weeks after end of treatment
References
Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81.
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74.
Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S206–14.
Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2007;37:676–91.
Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346–53.
Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. 2010;53:39–43.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–64.
Arase Y, Kumada H. Treatment strategy for chronic hepatitis C in Japan. Nihon Rinsho. 2011;69(Suppl 4):180–4.
Izumi N. Diagnostic and treatment algorithm of the Japanese society of hepatology: a consensus-based practice guideline. Oncology. 2010;78(Suppl 1):78–86.
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82.
Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55.
Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–17.
Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–16.
McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–303.
Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16.
Hayashi N, Okanoue T, Tsubouchi H, Toyota J, Chayama K, Kumada H. Efficacy and safety of telaprevir, a new protease inhibitor, for difficult-to-treat patients with genotype 1 chronic hepatitis C. J Viral Hepat. 2012;19:e134–42.
Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78–84.
Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206.
Hezode C. Boceprevir and telaprevir for the treatment of chronic hepatitis C: safety management in clinical practice. Liver Int. 2012;32(Suppl 1):32–8.
Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–24.
Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I et al. TMC435 in combination with peginterferon and ribavirin in treatment-naïve HCV genotype 1 patients: final analysis of the PILLAR phase IIb study. In: Abstract presented at the American Association for the Study of Liver Disease (AASLD), San Francisco, CA, USA, 4–8 November, 2011.
Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once-daily TMC435 plus peginterferon/ribavirin in hepatitis C genotype-1 patients: a randomized trial. Antivir Ther. 2011;16:1021–33.
Sekar V, Vis P, Lenz O, Meyvisch P, Peeters M, De Smedt G. Pharmacokinetic-pharmacodynamic (PK-PD) analyses of TMC435 in treatment-naive hepatitis C (HCV)-infected patients in the OPERA-1 study. Poster 1075 presented at the 45th Annual Meeting of the European Association for the Study of the Liver, 14–18 April, Vienna, Austria, 2010.
Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW et al. TMC435 with peginterferon and ribavirin in treatment-experienced HCV genotype 1 patients: the ASPIRE study, a randomised Phase IIb trial. Abstract A-455-0022-00482 presented at the Annual Meeting of the European Association for the Study of the Liver (EASL), Barcelona, Spain, 18–22 April, 2012.
Verloes R, Shishido A (2009) Phase I safety and PK of TMC435 in healthy volunteers and safety, PK and short-term efficacy in chronic hepatitis C infected individuals. Abstract O-32 presented at the Japanese Hepatology Congress, Kobe, Japan, 4–5 June, 2009.
Reddy KR, Lin F, Zoulim F. Response-guided and -unguided treatment of chronic hepatitis C. Liver Int. 2012;32(Suppl 1):64–73.
Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, et al. A matched case-controlled study of 48 and 72 weeks of peginterferon plus ribavirin combination therapy in patients infected with HCV genotype 1b in Japan: amino acid substitutions in HCV core region as predictor of sustained virological response. J Med Virol. 2009;81:452–8.
Lenz O, Vijgen L, Lin T-I, Verbinnen T, Berke JM, Meyvisch P, Simmen K, Fanning G. Analysis of the NS3 region in HCV genotype 1-infected patients treated with 5-day monotherapy of TMC435 in a Phase 1 study. In: 16th International Symposium on Hepatitis C Virus and Related Viruses, 3–7 October, Nice, France 2009; Poster P-247.
Huisman MT, Snoeys J, Monbaliu J, Martens M, Sekar V, Raoof A. In vitro studies investigating the mechanism of interaction between TMC435 and hepatic transporters. Poster 278 presented at the 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, USA, October 29-November 2, 2010.
Acknowledgments
The authors would like to thank all patients, their families, investigators and their staff in the following 25 study sites for completion of the study. The principal investigators for the TMC435-C215 study sites are listed below (alphabetical order): Akio Ido (Kagoshima University Medical and Dental Hospital), Eiji Mita (National Hospital Organization Osaka National Hospital), Harumasa Yoshihara (Osaka Rosai Hospital), Hideki Hagiwara (Kansai Rosai Hospital), Hideyuki Nomura (Shin-Kokura Hospital), Hiromitsu Kumada (Toranomon Hospital), Hiroshi Yatsuhashi (National Hospital Organization, Nagasaki Medical Center), Kazuhiro Katayama (Osaka Medical Center for Cancer and Cardiovascular Diseases), Kazushi Numata (Yokohama City University Medical Center), Kiyohide Kioka (Osaka City General Hospital), Kiyomi Yasuda (Kiyokawa Hospital), Masatoshi Kudo (Kinki University Hospital), Namiki Izumi (Musashino Red Cross Hospital), Norifumi Kawada (Osaka City University Hospital), Syouichi Takahashi (Hiroshima University Hospital), Syuhei Nishiguchi (The Hospital of Hyogo College of Medicine), Takayoshi Ito (Showa University Hospital), Takeji Umemura (Shinshu University Hospital), Tatsuya Ide (Kurume University Hospital), Tetsuo Takehara (Osaka University Hospital), Toshifumi Ito (Osaka Koseinenkin Hospital), Toshihide Shima (Saiseikai Suita Hospital), Yoichi Hiasa (Ehime University Hospital), Yoshito Itoh (University Hospital, Kyoto Prefectural University of Medicine), Yoshiyasu Karino (Sapporo Kosei General Hospital, Hokkaido). The DRAGON study was funded by Janssen Pharmaceutical K.K. Medical writing support was provided by Bethan Hahn, PhD, at Complete Medical Communications, and was funded by Janssen Research & Development.
Conflict of interest
Norio Hayashi has no conflict of interest. Chiharu Seto, Mai Kato, Yuji Komada, and Shoichiro Goto are employees of Janssen Pharmaceutical K.K.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hayashi, N., Seto, C., Kato, M. et al. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol 49, 138–147 (2014). https://doi.org/10.1007/s00535-013-0875-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0875-1