Abstract
Purpose
Patients undergoing treatment for cancer often report problems with their cognitive function, which is an essential component of health-related quality of life. Pursuant to this, a two-arm randomized, placebo-controlled, double-blind, phase III clinical trial was conducted to evaluate Ginkgo biloba (EGB 761) for the prevention of chemotherapy-related cognitive dysfunction in patients with breast cancer.
Methods
Previously chemotherapy naïve women about to receive adjuvant chemotherapy for breast cancer were randomized to receive 60 mg of EGB 761 or a matching placebo twice daily. The study agent was to begin before their second cycle of chemotherapy and to be taken throughout chemotherapy and 1 month beyond completion. The primary measure for cognitive function was the High Sensitivity Cognitive Screen (HSCS), with a secondary measure being the Trail Making Tests (TMT) A and B. Subjective assessment of cognitive function was evaluated by the cognitive subscale of the Perceived Health Scale (PHS) and the Profile of Mood States (POMS). Data were collected at baseline and at intervals throughout and after chemotherapy, up to 24 months after completion of adjuvant treatment. The primary statistical analysis included normalized area under the curve (AUC) comparisons of the HSCS, between the arms. Secondary analyses included evaluation of the other measures of cognition as well as correlational analyses between self-report and cognitive testing.
Results
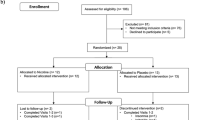
One hundred and sixty-six women provided evaluable data. There were no significant differences in AUC up to 12 months on the HSCS between arms at the end of chemotherapy or at any other time point after adjuvant treatment. There were also no significant differences in TMT A or B at any data point. Perceived cognitive functions, as measured by the PHS and confusion/bewilderment subscale of the POMS, were not different between arms at the end of chemotherapy. There was also little correlation between self-reported cognition and cognitive testing. No differences were observed in toxicities per Common Terminology Criteria for Adverse Events (CTCAE) assessment between Ginkgo biloba and placebo throughout the study; however, after chemotherapy, the placebo group reported worse nausea (p = .05).
Conclusion
This study did not provide any support for the notion that Ginkgo biloba, at a dose of 60 mg twice a day, can help prevent cognitive changes from chemotherapy. These analyses do provide data to further support the low associations between patients' self-report of cognition and cognitive performance, based on more formal testing.

Similar content being viewed by others
References
Huria A, Somlo G, Ahles T (2007) Renaming “chemobrain”. Cancer Investig 25:373–7
Pullens MJ, De Vries J, Roukema JA (2009) Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology
Wieneke M, Dienst E (1995) Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology 4:61–66
Brezden CB, Phillips KA, Abdolell M et al (2000) Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695–701
Castellon SA, Ganz PA, Bower JE et al (2004) Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26:955–69
Ferguson RJ, Ahles TA (2003) Low neuropsychologic performance among adult cancer survivors treated with chemotherapy. Curr Neurol Neurosci Rep 3:215–22
Kreukels BP, van Dam FS, Ridderinkhof KR et al (2008) Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin Breast Cancer 8:80–7
Schagen SB, van Dam FS, Muller MJ et al (1999) Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85:640–50
van Dam FS, Schagen SB, Muller MJ et al (1998) Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210–8
Kohli S, Griggs J, Roscoe JA et al (2007) Self-reported cognitive impairment in cancer patients. J Oncol Pract
Bower JE (2008) Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol 26:768–77
Janz NK, Mujahid M, Chung LK et al (2007) Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 16:1348–61
Mehnert A, Scherwath A, Schirmer L et al (2007) The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns 66:108–18
Mar Fan HG, Clemons M, Xu W et al (2008) A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 16:577–83
Nelson CJ, Nandy N, Roth AJ (2007) Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat Support Care 5:273–80
Kohli S, Fisher SG, Tra Y et al (2009) The effect of modafinil on cognitive function in breast cancer survivors. Cancer 115:2605–16
Le Bars PL, Katz MM, Berman N et al (1997) A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA 278:1327–32
Oken BS, Storzbach DM, Kaye JA (1998) The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol 55:1409–15
Natural Standard: Natural Standard Database
Schultz VHR, Tyler V (1998) Rational physotherapy: a physicians guide to herbal medicine, natural standard. Springer, New York, pp 38–49
Nada SE, Shah ZA (2012) Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis 46:180–9
Smith JV, Luo Y (2004) Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol 64:465–72
Faust D, Fogel BS (1989) The development and initial validation of a sensitive bedside cognitive screening test. J Nerv Ment Dis 177:25–31
Nelson A, Fogel BS, Faust D (1986) Bedside cognitive screening instruments. A critical assessment. J Nerv Ment Dis 174:73–83
Army Individual Test Battery (1944) Manual of direction and scoring. Washington, DC, War Department Adjutant General's Office
Fakouri C, Lyon B (2005) Perceived health and life satisfaction among older adults: the effects of worry and personal variables. J Gerontol Nurs 31:17–24
Barton D (2002) A model to predict negative health outcomes during cancer treatment
Dodge HH, Zitzelberger T, Oken BS et al (2008) A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology 70:1809–17
Snitz BE, O'Meara ES, Carlson MC et al (2009) Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA 302:2663–70
Solomon PR, Adams F, Silver A et al (2002) Ginkgo for memory enhancement: a randomized controlled trial. JAMA 288:835–40
Kanowski S, Herrmann WM, Stephan K et al (1996) Proof of efficacy of the Ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry 29:47–56
Donovan KA, Small BJ, Andrykowski MA et al (2005) Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer 104:2499–507
Tannock IF, Ahles TA, Ganz PA et al (2004) Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol 22:2233–9
Vardy J, Wong K, Yi QL et al (2006) Assessing cognitive function in cancer patients. Support Care Cancer 14:1111–8
Kibiger G, Kirsh KL, Wall JR et al (2003) My mind is as clear as it used to be: a pilot study illustrating the difficulties of employing a single-item subjective screen to detect cognitive impairment in outpatients with cancer. J Pain Symptom Manage 26:705–15
Cull A, Hay C, Love SB et al (1996) What do cancer patients mean when they complain of concentration and memory problems? Br J Cancer 74:1674–9
Poppelreuter M, Weis J, Kulz AK et al (2004) Cognitive dysfunction and subjective complaints of cancer patients. a cross-sectional study in a cancer rehabilitation centre. Eur J Cancer 40:43–9
Ferguson RJ, McDonald BC, Saykin AJ et al (2007) Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol 25:3866–70
McDonald BC, Conroy SK, Ahles TA et al (2010) Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat 123:819–28
Penttinen HM, Saarto T, Kellokumpu-Lehtinen P, et al (2010) Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psychooncology
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported, in part, by Public Health Service grants CA-25224, CA-37404, CA-63849, CA-35195, CA-35431, CA-35269, CA-35101, CA-63848, CA-37417, CA-35415, CA-35448, CA-35103, CA-35119, CA-35103, and CA-35272. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additional participating institutions include: Siouxland Hematology-Oncology Associates, Sioux City, IA 51105, USA (Donald Wender, M.D.); Toledo Community Hospital Oncology Program (Paul L. Schaefer, M.D.); Medical College of Georgia, Augusta, GA 30912, USA (Anand P. Jillella M.D.); Iowa Oncology Research Association CCOP, Des Moines, IA 50309, USA (Robert J. Behrens, M.D.); Rapid City Regional Hospital, Inc., Rapid City, SD 57701, USA (Richard Charles Tenglin, M.D.); Columbus CCOP, Columbus, OH 53215, USA (J. Philip Kuebler, M.D., Ph.D.); Mayo Clinic Florida, Jacksonville, FL 32224, USA (Kurt A. Jaeckle, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106, USA (Philip J. Stella, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122, USA (Preston D. Steen, M.D.); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822,USA (Albert M. Bernath, Jr, M.D.); Upstate Carolina CCOP, Spartanburg, SC 29303, USA (James D. Bearden, III, M.D.); Montana Cancer Consortium CCOP, Billings, MT 59101, USA (Benjamin T. Marchello, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105, USA (Miroslaw Muzurczak, M.D.); Lehigh Valley Hospital, Allentown, PA 18103, USA (Suresh Nair, M.D.); Ochsner CCOP, New Orleans, LA 70121, USA (Jyotsna Fuloria, M.D.); Colorado Cancer Research Program, Denver, CO 80224, USA (Eduardo R. Pajon, Jr., M.D.).
Conflict of interest
None of the authors have any conflict of interest. Schwabe did supply Ginkgo biloba and placebo for this study, but none of the authors have any financial relationship with them. Our biostatisticians had full control of all primary data throughout data collection and analysis and still have these data under their control.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barton, D.L., Burger, K., Novotny, P.J. et al. The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support Care Cancer 21, 1185–1192 (2013). https://doi.org/10.1007/s00520-012-1647-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1647-9